
BD offers a variety of Extension Sets with various needle-free connectors that help meet clinical IV needs throughout the hospital.

BD MaxPlus™ Extension Sets
The BD MaxPlus™ connector is the only needle-free connector with an FDA-cleared label statement demonstrating a reduction in CLABSI.¹
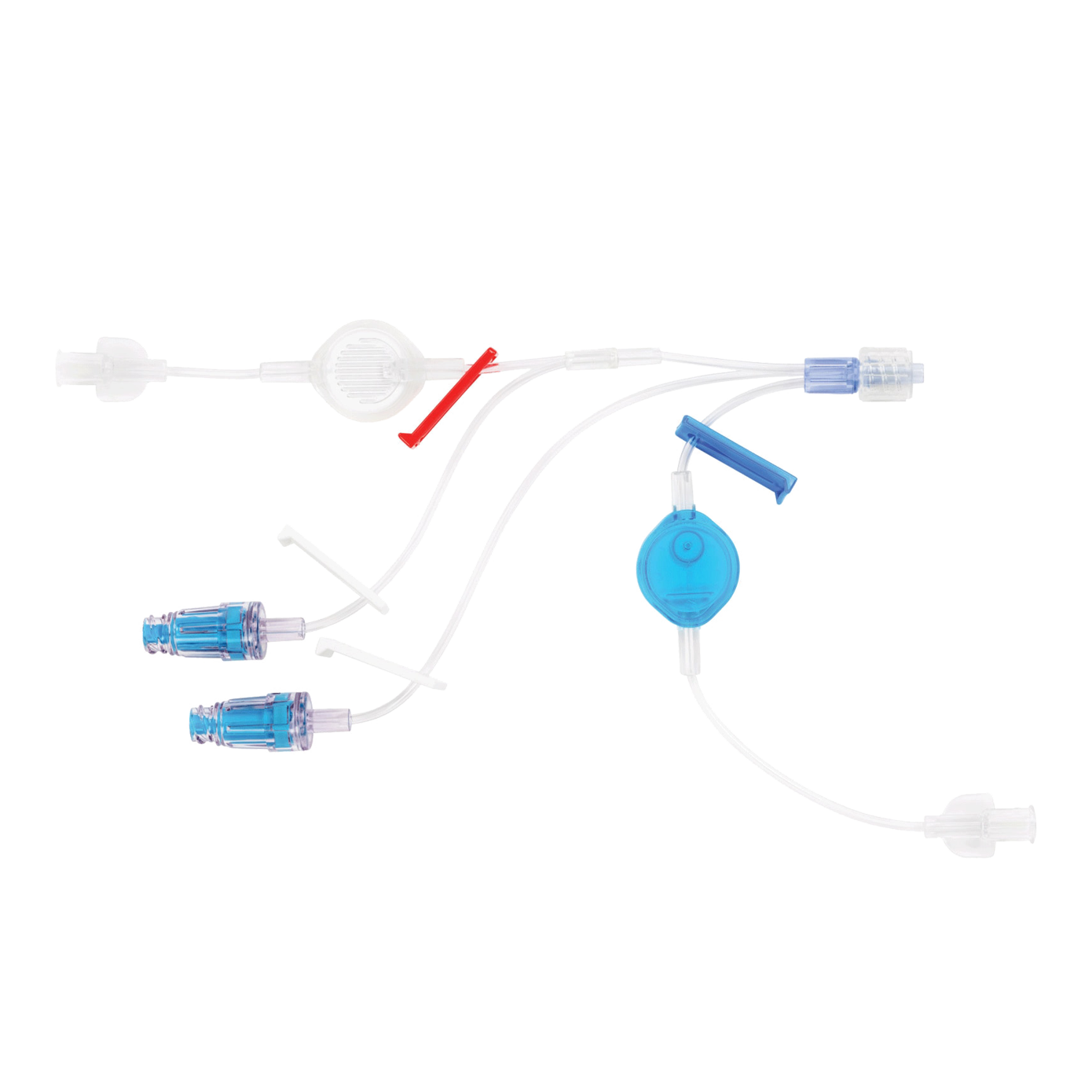
BD MaxZero™ Extension Sets
The BD MaxZero™ needle-free connector utilizes the proven technology of the BD MaxPlus™.*

BD NeutraClear™ Extension Sets
The BD NeutraClear™ needle-free connector has low weight, low priming volume and compact size, that makes it suitable for neonate and paediatric use.
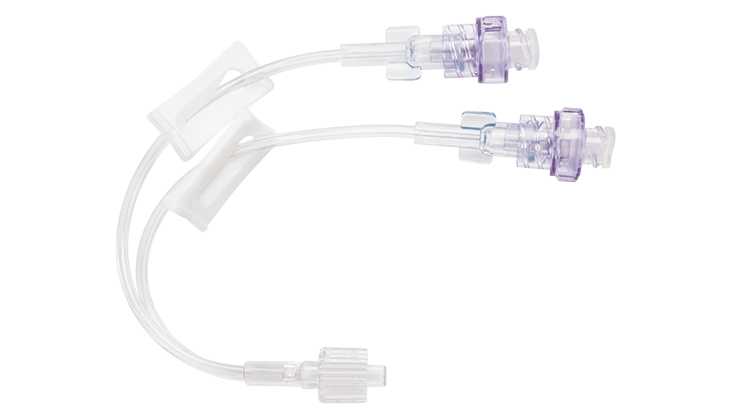
BD Q-Syte™ Extension Sets
The BD Q-Syte™ luer access split septum features a simple internal design with a straight, open fluid path for high flow rate clinical practice needs.
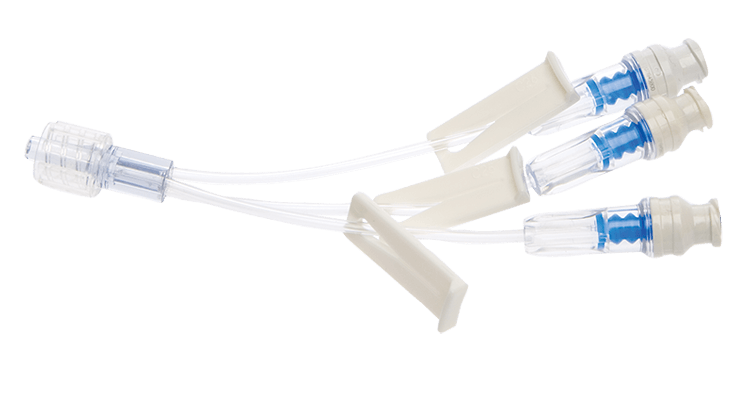
BD SmartSite™ Extension Sets
Our BD SmartSite™ Extension Sets come in a variety of configurations to meet your diverse clinical needs.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes:
* The BD MaxPlus™ needle-free connector and the BD MaxZero™ needle-free connector use the same technology: the BD Max needlefree connector technology. Data on file: REF-3210 - 510(k), 132413, Section 11.1: Device Description, page 43 (6 Feb 2018).
References
- Data on File: REF-3786 - PS100009 and PS100010 / 510(k) Number: K051939 and K072542 (6 Aug 2014).