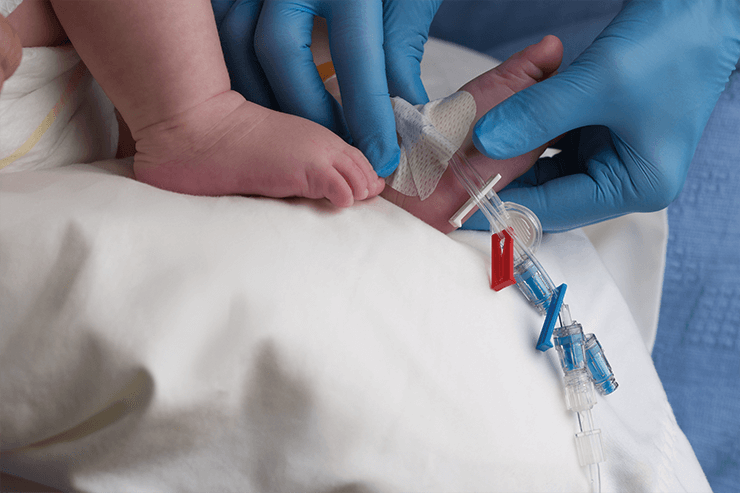
The BD MaxZero™ needle-free connector leverages the same technology as the BD MaxPlus™ connector*, designed to help reduce the risk of infections and occlusions. Its low weight and low priming volume compared to BD MaxPlus™ make it especially suitable for use in all patients, including infants and children.
- Overview
- EIFU & Resources

Solid, sealed access surface
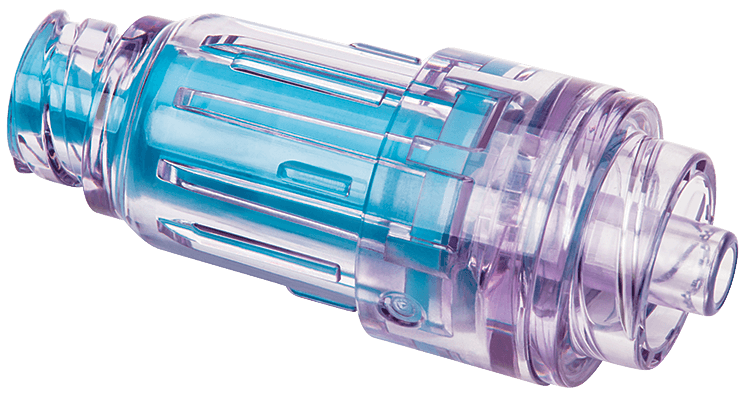
Clear fluid path
A clear fluid housing enables clinicians to visualise the fluid path easily.

Proven technology
Same core technology as the MaxPlus™ Needleless Connector, which has shown superior results in preventing the risk of infection.¹
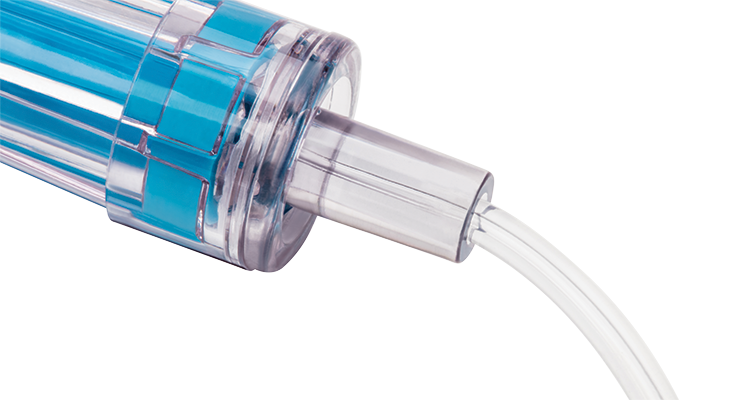
Zero reflux upon disconnection
The positive displacement helps reduce catheter occlusions and their associated costs and risks.³
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes:
* The BD MaxPlus™ needle-free connector and the BD MaxZero™ needle-free connector use the same technology: the BD Max needlefree connector technology. Data on file: REF-3210 - 510(k), 132413, Section 11.1: Device Description, page 43 (6 Feb 2018).
References
- Tabak YP, Johannes RS, Sun X, Crosby CT, Jarvis WR. Innovative use of existing public and private data sources for postmarket surveillance of central line-associated bloodstream infections associated with intravenous needleless connectors. J Infus Nurs. 2016;39(5):328-335.
- Data on File: REF-2224 - An extended microbial challenge study comparing two needle-free connectors: BD MaxZero™ and MicroClave® needle-free connectors (18 Sep 2017).
- Williams A. Catheter occlusion in-home infusion: the influence of needleless connector design on central catheter occlusion. J Infus Nurs. 2018;41(1):52-57.
BD-39472 (08/2021)