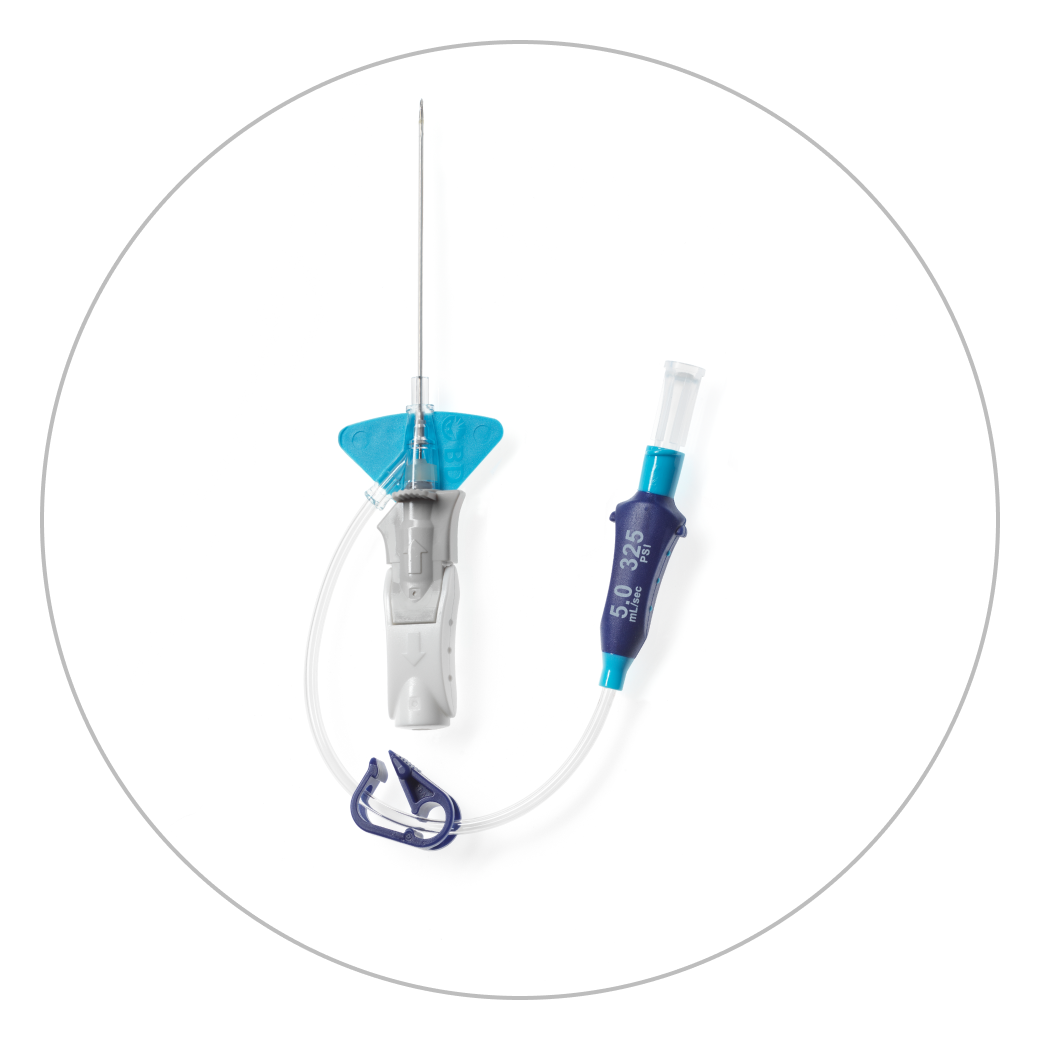
The BD Nexiva™ Diffusics™ Closed IV Catheter System is built upon the BD Nexiva™ Diffusics™ Closed IV Catheter System with the added benefit of a diffusion catheter tip designed to address common IV challenges in CT, such as sufficient gauge size and catheter stability in the vein, during power injection procedures.

- BD Nexiva Diffusics™ Catheter System
- Cardiac CTs
- Clinical Evidence
- Product Features
- Products and Accessories
- EIFU & Resources
High flow rates have traditionally required large gauge catheters
Unfortunately, many patients’ vasculature cannot accommodate 18- or even 20-gauge catheters, and using smaller gauge sizes can result in slower flow rates and reduced image quality.
High flow rates may cause catheters to whip around in the vein.
While achieving appropriate flow rates is crucial to image quality, the high flow rates required for power injection during CT may also cause catheters to move or whip around in the vein which may lead to extravasation and could be costly.1,2
Enable higher flow rates with smaller gauges
The Infusion Therapy Standards of Practice recommend placing the smallest-gauge catheter that will accommodate the prescribed therapy and patient need.3
The diffusion tip on Nexiva Diffusics™ Catheter System enables desired contrast media flow rates to be achieved using a smaller gauge catheter† without compromising image quality. This enables clinicians to perform CT scans that require high flow rates when a patient's vasculature cannot accommodate a large catheter (i.e., 18 or 20 gauge).‡,4
†22–24 gauge
‡Bench test results may not necessarily be indicative of clinical performance.
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Reduce the force of contrast delivery
While flow rates of contrast media are important, the delivery force should also be considered. Nexiva Diffusics™ Catheter System’s diffusion tip reduces the force of contrast delivery by up to 48%,§ helping decrease the impact of contrast media on the vessel wall.
§Compared to a non-diffusion-tip catheter. Bench test results may not necessarily be indicative of clinical performance.
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
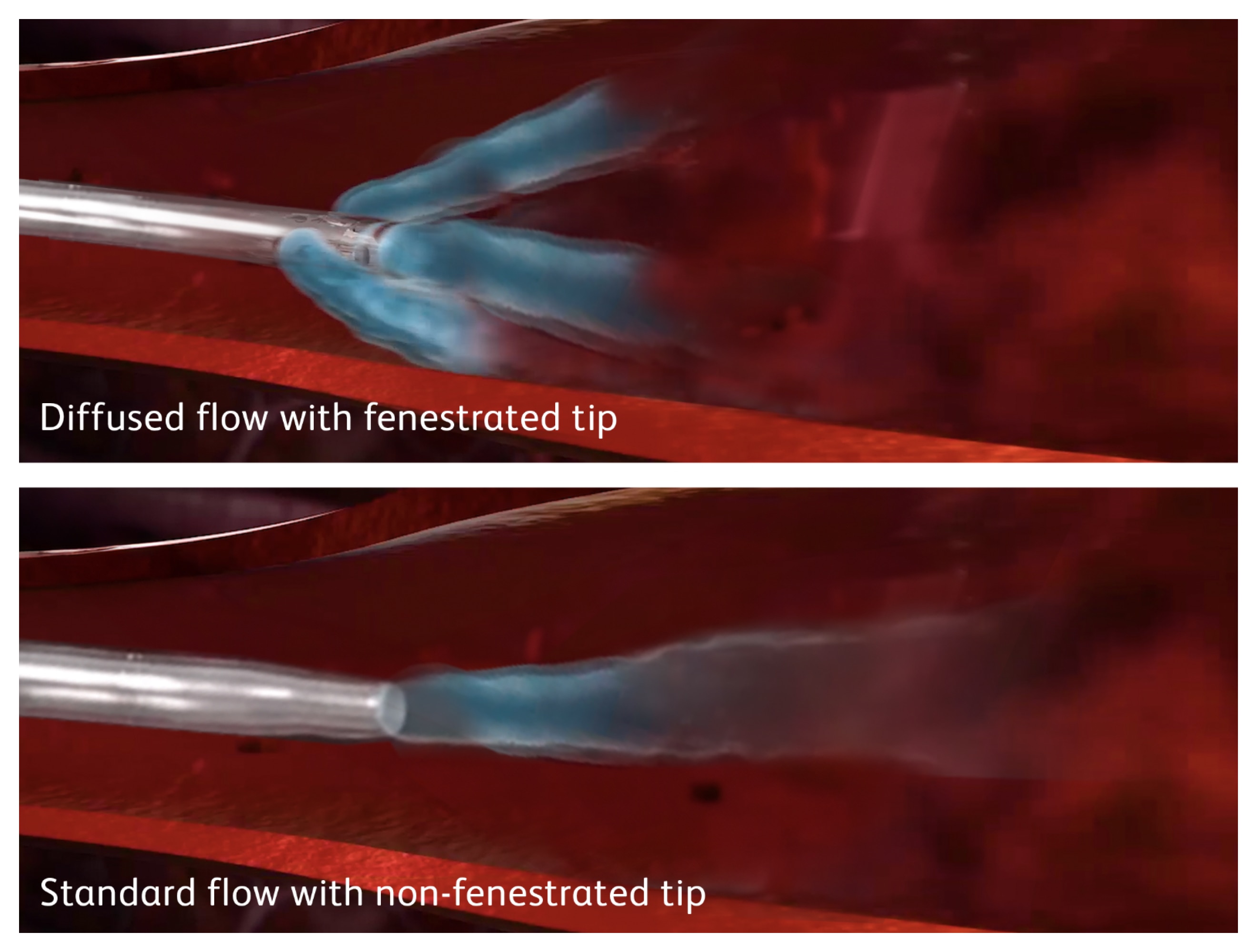
Decrease potential catheter motion in vein
High flow rates required for power injection during CT may cause some catheters to move or whip around in the vein. Catheter motion may lead to extravasation, a complication caused by the catheter backing out of the vein.1
Extravasation may be painful for the patient, potentially resulting in tissue damage.3 The average potential liability for a moderate extravasation is $22k and up to $145k for a severe extravasation.2
Nexiva Diffusics™ Catheter System has been shown to reduce the forces that may cause catheter motion in the vein by up to 67%. This reduction in force stabilizes the catheter in the vein, and prevents the whipping, movement and backing out of the vein that may lead to extravasation.1
The videos below show catheter motion and extravasation of a non-diffusion tip catheter compared to the stability that Nexiva Diffusics™ Catheter System provides during power injection.
Power injection through a non-fenestrated catheter: Extravasation
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Note that, due to the force of the injection, the catheter tip will slowly move, backing out of the vein until the tip escapes the vein completely and contrast media enters the surrounding tissue.
Power injection through a non-fenestrated catheter: Catheter Motion
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Note the catheter stays in the vein, but the forces from the power injection create enough turbulence to cause the catheter tip to whip around within the vein. This creates significant motion, potentially irritating or damaging the vessel wall.
Power injection through Nexiva Diffusics™ Catheter System with fenestrated tip: Stability
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Note the contrast media flows throughout the vasculature, while the catheter remains still.
†Videos are from an animal study. Animal study results may not be indicative of clinical performance.
Nexiva Diffusics™ Catheter System Portfolio
Nexiva Diffusics™ Catheter System is offered in two single port configurations: with and without BD MaxZero™ Needle-free Connectors.
- Boschi R, Rostagno R. Extravasation of antineoplastic agents: prevention and treatments. Pediatric Reports. 2021;4(e28);98-100.
- Paice T. Economic impact of an extravasation. an analysis. Imaging Economics. 2007;20(3):14.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2024.
- Johnson PT, Christensen GM, Fishman EK. IV contrast administration with dual source 128-MDCT: a randomized controlled study comparing 18-gauge nonfenestrated and 20-gauge fenestrated catheters for catheter placement success, infusion rate, image quality and complications. Am J Roentgenol. 2014;202(6):1166-70.
BD-22672 (05/24)
Nexiva Diffusics™ Catheter System for cardiac CTs
The all-in-one closed IV catheter system leverages a fenestrated tip to accommodate power injection at high flow rates (e.g., 6.5 mL/sec for 22 G) and provides higher vascular attenuation for coronary CTA (CCTA) when compared with non-fenestrated catheters at the same flow rates.
Practice guidelines include using a fenestrated peripheral IV catheter (PIVC), ensuring the IV used is rated to administer flow rates required for coronary CTA (e.g., 5–7 mL/s).1
-

ensures high risk patients are appropriately triaged to cardiac catheterization1
-

offers rapid evaluation of the degree of coronary stenosis and atherosclerosis1
-

allows significant reduction in time-to-discharge1
-
Appropriate utilization and patient selection1
-
Requires high contrast media injection flow rates2
Extracting potential clinical and economic value for CCTA includes improving patient outcomes, lowering healthcare costs and preventing unnecessary diagnostic procedures.1
Watch a free webinar featuring Dr. Wesley T. O’Neal, MD, FACC, Medical Director, Cardiovascular CT Cone Health Heart & Vascular Center of Greensboro NC
Elevate infusion experiences for your patients
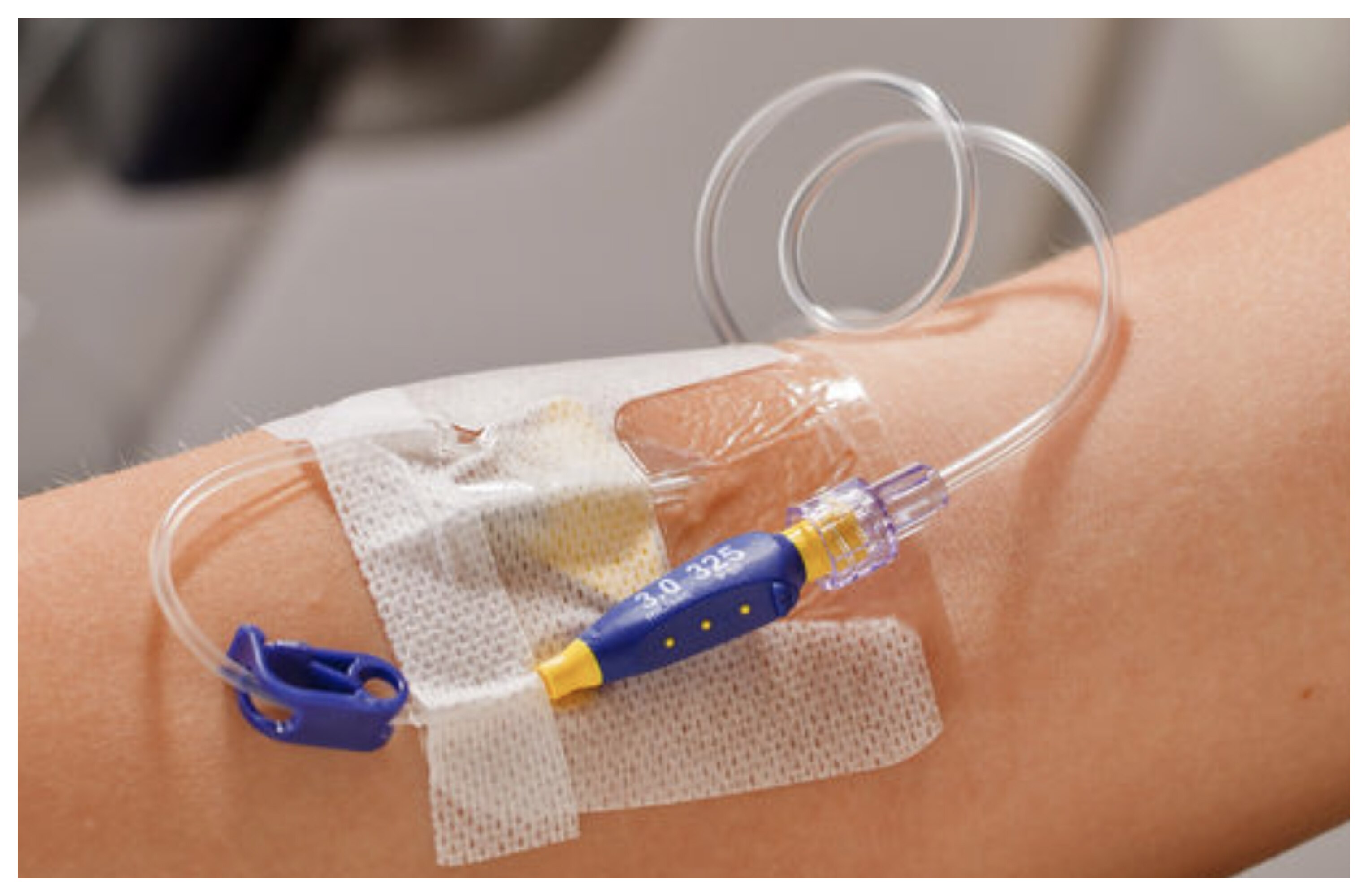
Fenestrated catheter in CCTA1,2
Nexiva Diffusics™ Catheter System Portfolio
Nexiva Diffusics™ Catheter System is offered in two single port configurations: with and without BD MaxZero™ Needle-free Connectors.
- Maroules C, Rybicki F, Ghoshhajra B, et al. 2022 use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department. Journal of Cardiovascular Computed Tomography. 2023;17:146-163. https://doi.org/10.1016/j.jcct.2022.09.003.
- Fischer AM, Riffel P, Henzier T, et al. More holes, more contrast? Comparing an 18-gauge non-fenestrated catheter with a 22-gauge fenestrated catheter for cardiac CT. PLoS ONE 15(6):e0234311. https://doi.org/10.1371/journal.pone.0234311
- Boschi R, Rostagno R. Extravasation of antineoplastic agents: prevention and treatments. Pediatric Reports. 2021;4(e28);98-100.
- Cademartiri F, Mollet N, Lemos P, et al. Higher intracoronary attenuation improves diagnostic accuracy in MDCT coronary angiography. AJR. 2006;187:W430-W433. doi:10.2214/AJR.05.1406
- Harraz M, Abouissa A, Kamr W. Closed versus conventional IV catheter in performing coronary CT Angiography. Egyptian Journal of Radiology and Nuclear Medicine. (2020) 51:89. https://doi.org/10.118/s43055-020-00211-4.
- Kim JeongJae, Kim Eun Jeong, Hur Jee Hye, et al. The Usefulness of Fenestrated Intravenous Catheters Compared with Nonfenestrated Catheter for Cardiac Multidetector Computed Tomography. J Comput Assist Tomogr. Volume 43, Number 3, May/June 2019.
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2024.
Clinical evidence
Nexiva Diffusics™ Catheter System is built on the Nexiva™ platform. Nexiva™ has been shown to deliver higher clinician satisfaction, improved clinician safety and a reduction in overall complication rates versus non-integrated, open systems.1–3
The platform is backed by evidence in peer-reviewed journals from as early as 20094 to as recently as 2024.5
Nexiva™ Diffusics Closed IV Catheter System is also backed by peer-reviewed journals and infusion guidelines. Infusion Therapy Standards of Practice state to consider a fenestrated catheter for a contrast-based radiographic study and in coronary CTA.6 Nexiva Diffusics™ Catheter System is the only peripheral IV catheter with a diffusion catheter tip specifically designed for power injection.
Closed IV Catheter System | Comparative catheter | Data gathered | Year published | Study type | Journal | Authors |
| Nexiva Diffusics™ | Smiths Jelco ProtectIV® Plus | Aortic enhancement levels, catheter placement success, complications, contrast volume, extravasation, image quality, infusion rate, maximum pressure | 2014 | RCT7 | American Journal of Roentgenology | Johnson PT, Christensen GM, Fishman EK. |
| Nexiva™ | Various | Catheter failure, dislodgement, occlusion, phlebitis | 2024 | Systematic review/ meta analysis5 | Journal of Vascular Access | Gidaro A, Quici M, Giustivi D, et al. |
| Nexiva™ | BD Insyte Autoguard™ | Dwell time, complications, dislodgement, occlusion, infiltration, phlebitis | 2023 | RCT8 | European Journal of Oncology Nursing | Gerceker GO,
Yildirim BG, Onal A, et al. |
| Nexiva™ | B. Braun
Introcan
Safety® BD Insyte Autoguard™ | Catheter-associated bloodstream infection, clinician satisfaction, cost savings/utilization reduction, dwell time, dislodgement/leaking, infiltration/extravasation, local infection, insertion success, occlusion, phlebitis | 2022 | RCT1 | Journal of Hospital Medicine | Rickard CM,
Larsen E, Walker RM, et al. |
| Nexiva™ | Smiths Jelco ProtectIV® Plus | Clinician satisfaction, dwell time, infection, infiltration, pain, phlebitis | 2019 | Prospective, quasi-experimental9 | Journal of Infusion Nursing | Penoyer D, Fowler S, Bennett M |
| Nexiva™ | None | Blood exposure, dislodgement, kinking, leaking, phlebitis, restarts | 2017 | Observational10 | Journal of Vascular Access | deRosenroll A |
| Nexiva™ | Medikit | Bending and kinking, blood exposure, cost savings/utilization reduction, dislodgement, dwell time, extravasation, insertion success, obstruction, phlebitis | 2014 | Quasi-randomized study11 | Journal of Vascular Access | Tamura N, Abe S, Hagimoto K, et al. |
| Nexiva™ | B. Braun Vasocan® Safety | Catheter-related infection, cost savings/utilization reduction, dwell time, infiltration/extravasation, insertion success, occlusion, pain, painful hematoma, phlebitis | 2014 | RCT2 | Journal of Hospital Infection | González López J, Arribi Vilela A, Fernández Del, Palacio E, et al. |
| Nexiva™ | B. Braun Introcan Safety® (non-winged) with StatLock | Clinician satisfaction, cost savings/utilization reduction, dislodgement, dwell time, infiltration, insertion success, leaking, phlebitis | 2010 | RCT3 | Journal of Infusion Nursing | Bausone-Gazda D, Lefaiver CA, Walters SA, et al. |
| Nexiva™ | None | Clinician satisfaction, clotting, dislodgement, infiltration, leaking, restarts, securement | 2009 | Pre-and post-use survey4 | Journal of the Association of Vascular Access | McNeil E, Hines N, Phariss R |
- Rickard CM, Larsen E, Walker Rachel M, et al. Integrated versus nonintegrated peripheral intravenous catheter in hospitalized adults (OPTIMUM): A randomized controlled trial. J Hosp Med. 2022;1-12.
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- McNeil E, Hines N, Phariss R. A clinical trial of a new all-in-one peripheral-short catheter. JAVA. 2009;14(1):46-51.
- Gidaro A, Quici M, Giustivi D, et al. Integrated short peripheral intravenous cannulas and risk of catheter failure: a systematic meta-analysis. J Vasc Access. 2024.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2024.
- Johnson PT, Christensen GM, Fishman EK. IV contrast administration with dual source 128-MDCT: a randomized controlled study comparing 18-gauge nonfenestrated and 20-gauge fenestrated catheters for catheter placement success, infusion rate, image quality and complications. Am J Roentgenol. 2014;202(6):1166-70.
- Gerceker GO, Yildirim BG, Onal A, et al. The effect of the closed intravenous catheter system on first insertion success, indwelling time, and complications in pediatric hematology and oncology patients: a randomized controlled study. Eur J Oncol Nurs. 2023 Dec:67:102430. doi: 10.1016/j.ejon.2023.102430. Epub 2023 Oct 7.
- Penoyer D, Fowler S, Bennett M. Evaluation of the Use of Open Versus Closed Short Peripheral Catheters on Catheter Dwell Time. J Infus Nurs. 2019;42(6):276-282.
- deRosenroll A. Peripheral intravenous catheters: Improving outcomes through change in products, clinical practice and education. Vasc Access. 2017;11(1):7-12.
- Tamura N, Abe S, Hagimoto K, et al. Unfavorable peripheral intravenous catheter replacements can be reduced using an integrated closed intravenous catheter system. J Vasc Access. 2014;15(4):257-263.
BD Nexiva Diffusics™ Catheter System

- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
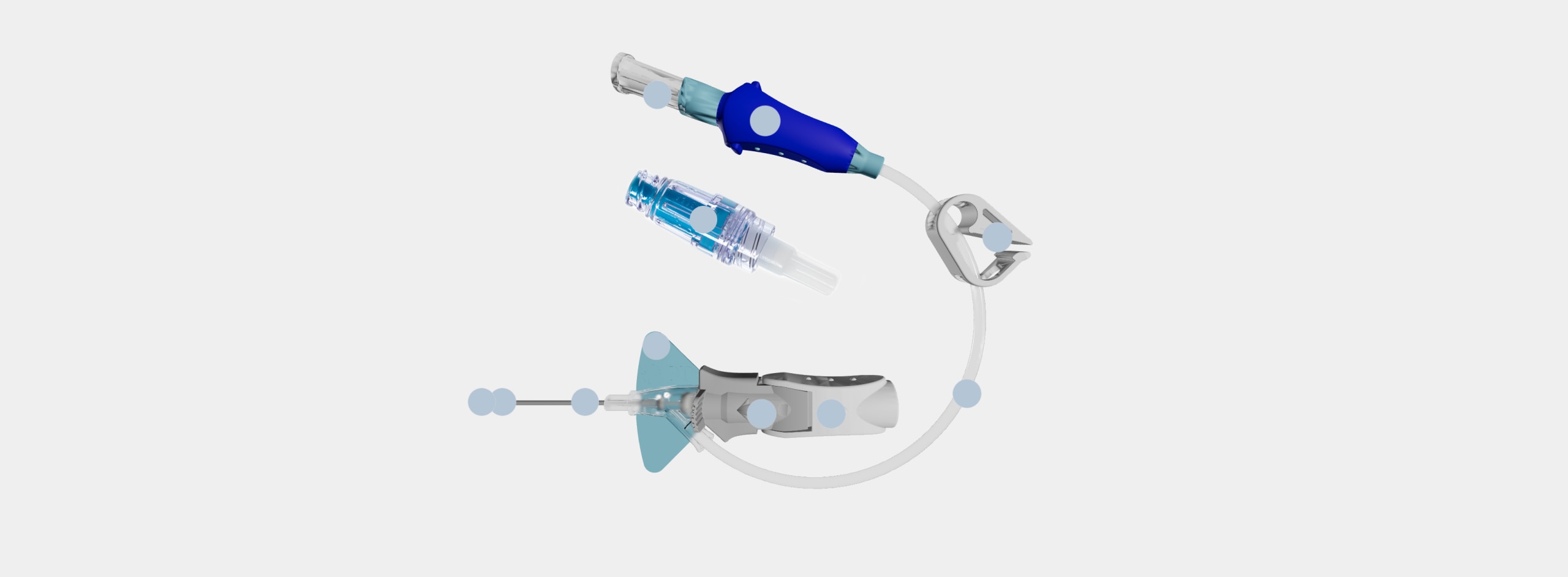
Vent plug
During insertion, the vent plug allows air to escape from the system enabling blood return to be seen in the catheter and extension tubing. The vent plug seals when blood reaches it, containing the blood within the system.
Single port
The port is designed for the administration of a single solution after attaching a needle-free connector.
Pinch clamp
The pinch clamp is used to control the flow of blood within the extension tubing and prevent blood leakage when replacing the vent plug or changing the needle-free connector. Each type of needle-free connector has a specified clamping sequence.
Pre-attached extension tube
The pre-attached extension tube, combined with the vent plug, creates a closed/integrated system that contains the blood as the catheter is inserted and serves as a secondary flashback chamber to support first attempt insertion success.
Ergonomic grip
The ergonomic grip is utilized during insertion and advancement of the catheter. After catheter advancement, pulling back on the white component releases the grey push tab component.
Passive safety mechanism
The passive safety mechanism fully encapsulates the needle tip as the needle is withdrawn and the gray push-tab component releases from the built-in stabilization platform. This helps reduce the risk of needlestick injury.
Stabilization platform
The built-in stabilization platform facilitates securement of the catheter to the patient and eliminates the need to purchase and add an external stabilization device, as is necessary with non-integrated PIVCs.
BD Vialon™ Catheter Material
The catheter resides within the vein and is made of a proprietary polyurethane named Vialon™ Catheter Material, which is differentiated from competitive polyurethane catheters and is utilized only in BD PIVCs.
BD Instaflash™ Needle Technology
Instaflash™ Needle Technology is the hole in the needle that allows blood to flow between the needle and the catheter so initial blood return is seen in the catheter. Blood continues to flow up the integrated extension set as confirmation of placement.
Diffusion tip technology
The diffusion tip technology is designed to address common IV challenges in CT, such as sufficient gauge size and catheter stability in the vein during power injection.
Catalog no. | Color | Gauge | Catheter length (in) | Gravity flow rate (mL/min) | Maximum power
| Max power
|
| 383590 | Yellow | 24 | 0.75 | 21 | 325 | 3.00 mL/sec |
| 383591 | Blue | 22 | 1.00 | 45 | 325 | 6.50 mL/sec |
| 383592 | Pink | 20 | 1.00 | 68 | 325 | 10.00 mL/sec |
| 383593 | Pink | 20 | 1.25 | 64 | 325 | 10.00 mL/sec |
| 383594 | Green | 18 | 1.25 | 90 | 325 | 15.00 mL/sec |
*The catheter system has been tested at flow rates; however, due to variations in add-on devices, tubing, contrast media temperature and pressure limit settings these flow rates may not be achievable.
BD Nexiva Diffusics™ Catheter System with BD MaxZero™ Needle-free Connectors
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
BD MaxZero™ Needle-Free Connector
Needle-free connectors close the system while enabling access for delivery of infusion therapy.
Needle-free connectors must be purchased separately from open/non-integrated PIVCs.
Vent plug
During insertion, the vent plug allows air to escape from the system enabling blood return to be seen in the catheter and extension tubing. The vent plug seals when blood reaches it, containing the blood within the system.
Single port
The port is designed for the administration of a single solution after attaching a needle-free connector.
Pinch clamp
The pinch clamp is used to control the flow of blood within the extension tubing and prevent blood leakage when replacing the vent plug or changing the needle-free connector. Each type of needle-free connector has a specified clamping sequence.
Pre-attached extension tube
The pre-attached extension tube, combined with the vent plug, creates a closed/integrated system that contains the blood as the catheter is inserted and serves as a secondary flashback chamber to support first attempt insertion success.
Ergonomic grip
The ergonomic grip is utilized during insertion and advancement of the catheter. After catheter advancement, pulling back on the white component releases the grey push tab component.
Passive safety mechanism
The passive safety mechanism fully encapsulates the needle tip as the needle is withdrawn and the gray push-tab component releases from the built-in stabilization platform. This helps reduce the risk of needlestick injury.
Stabilization platform
The built-in stabilization platform facilitates securement of the catheter to the patient and eliminates the need to purchase and add an external stabilization device, as is necessary with non-integrated PIVCs.
BD Vialon™ Catheter Material
The catheter resides within the vein and is made of a proprietary polyurethane named Vialon™ Catheter Material, which is differentiated from competitive polyurethane catheters and is utilized only in BD PIVCs.
BD Instaflash™ Needle Technology
Instaflash™ Needle Technology is the hole in the needle that allows blood to flow between the needle and the catheter so initial blood return is seen in the catheter. Blood continues to flow up the integrated extension set as confirmation of placement.
Diffusion tip technology
The diffusion tip technology is designed to address common IV challenges in CT, such as sufficient gauge size and catheter stability in the vein during power injection. The unique diffusion tip adds three teardrop-shaped holes to diffuse flow.
Catalog no. | Color | Gauge | Catheter
| Catheter
| Catheter
| Extension
| Gravity
| Max power
| Packaging |
| 383595 | Yellow | 24 | .75 | 0.0210 (0.53) | 0.0280 (0.71) | 1.22 | 21 | 3.0 | 20/box 80/ case |
| 383596 | Blue | 22 | 1.00 | 0.0265 (0.67) | 0.0355 (0.90) | 1.22 | 45 | 6.5 | 20/box 80/ case |
| 383598 | Blue | 22 | 1.75 | 0.0265 (0.67) | 0.0355 (0.90) | 1.65 | 33 | 5.0 | 20/box 80/ case |
| 383597 | Pink | 20 | 1.00 | 0.0325 (0.83) | 0.0435 (1.10) | 1.65 | 68 | 10.0 | 20/box 80/ case |
| 383587 | Pink | 20 | 1.25 | 0.0325 (0.83) | 0.0435 (1.10) | 1.65 | 64 | 10.0 | 20/box 80/ case |
| 383599 | Pink | 20 | 1.75 | 0.0325 (0.83) | 0.0435 (1.10) | 1.65 | 53 | 8.5 | 20/box 80/ case |
| 383588 | Green | 18 | 1.25 | 0.0385 (0.98) | 0.0515 (1.31) | 1.65 | 90 | 15.0 | 20/box 80/ case |
| 393589 | Green | 18 | 1.75 | 0.0385 (0.98) | 0.0515 (1.31) | 1.65 | 84 | 11.0 | 20/box 80/ case |
*The catheter system has been tested at flow rates; however, due to variations in add-on devices, tubing, contrast media temperature and pressure limit settings these flow rates may not be achievable.
Find our training resources to help improve your clinical practices as part of our goal of advancing the world of health.