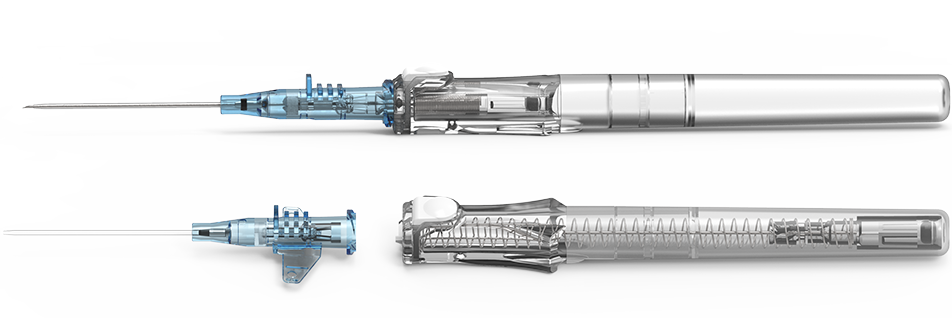
IV catheter placement is the most common invasive hospital procedure worldwide.1,2 Up to 90% of hospitalized patients require IV therapy, over 95% of these devices are peripheral IV catheters (PIVC).2 With every PIVC insertion attempt, healthcare workers (HCWs) risk exposure to patient blood.3-5 Needlestick injuries (NSIs) and blood leakage may expose HCWs to more than 30 dangerous pathogens that can be present in human blood, including hepatitis B, C and HIV.6 The BD Insyte Autoguard™ BC Shielded IV Catheter with blood control technology is designed to provide HCWs protection from needlestick injuries and blood exposure.
BD Insyte Autoguard™ BC Shielded IV Catheter with Blood Control Technology

- Overview
- Products & Accessories
- EIFU & Resources

Making IV access more convenient
No need to apply venous compression during initial insertion with BD Insyte Autoguard™ BC Shielded IV Catheters.7 BD Insyte Autoguard™ BC Shielded IV Catheters with blood control technology are designed to stop the flow of blood from the catheter hub until the initial luer connection is made.

Making IV access safer for healthcare workers
BD Insyte Autoguard™ BC Shielded IV Catheters with blood control technology demonstrated a 95% reduction in the risk of blood exposure.7*^

Making IV access less risky
In a healthy human study, 98% of clinicians indicated they felt no risk of blood exposure during insertion when using BD Insyte Autoguard™ BC Shielded IV Catheters.7*^

Making IV access more successful
BD Instaflash™ Needle Technology incorporates a notched needle, which was clinically demonstrated to improve first-attempt insertion success.8-9
- 1
- 2
- 3
- 4
- 5
- 6
BD Instaflash™ Needle Technology (now on all 18–24-gauge catheters)
Blood control technology is designed to stop the flow of blood from the catheter hub until the initial luer connection is made
Push-button needle shielding technology
Proprietary BD Vialon™ Catheter Material softens, enabling longer dwell time and reducing the chance of phlebitis
Tall push tab and three ridges designed to prevent rotation of the catheter hub during advancement
Complete needle encapsulation
- Helm RE, Klausner JD, Klemperer JD, et al. Accepted but unacceptable: peripheral IV catheter failure: Infus Nurs Society. 2015;38(3):189-203.
- iData Research Inc. Global Market Report Suite for Vascular Access Devices and Accessories. 2020.
- Tarantola A, Abiteboul D, Rachline A. Infection risks following accidental exposure to blood or body fluids in health care workers: a review of pathogens transmitted in published cases. Am J Infect Control. 2006:34:367-375.
- Haeseler G, Hildebrand M, Fritscher J. Efficacy and ease of use of an intravenous catheter designed to prevent blood leakage: a prospective observational trial. JVA. 2015;16(3):233-236. doi: 10.5301/jva.5000334.
- Jagger J Perry J, Parker G, et al. Survey results: Blood exposure risk during peripheral IV catheter insertion and removal. Nursing. 2011;41(12):45-49. doi:10.1097/01.NURSE.0000407678.81635.62
- European Biosafety Network. Prevention of Sharps Injuries in the Hospital and Healthcare Sector. European Biosafety Network Implementation Guidance Toolkit for EU Council Directive. 2010/32/EU. January 2013. Available at www.europeansafetynetwork.eu (accessed May 2017).
- Onia R, Eshun-Wilson I, Arce C, et al. Evaluation of a new safety peripheral IV catheter designed to reduce mucocutaneous blood exposure. CMRO. 2011;27(7):1339-1346.
- van Loon FHJ, Timmerman R, den Brok GPH, Korsten EHM, Dierick-van Daele ATM, Bouwman ARA. The impact of a notched peripheral intravenous catheter on the first attempt success rate in hospitalized adults: block-randomized trial. JVA. 2021 doi: 10.1177/1129729821990217.
- Seetharam AM, Raju U, Suresh K. A randomized controlled study to compare first stick success with Instaflash technology: The FIRSST study. JVA. 2022 doi:10.1177/11297298221080369.
BD-14385 (02/24)
Find our training resources to help improve your clinical practices as part of our goal of advancing the world of health.

