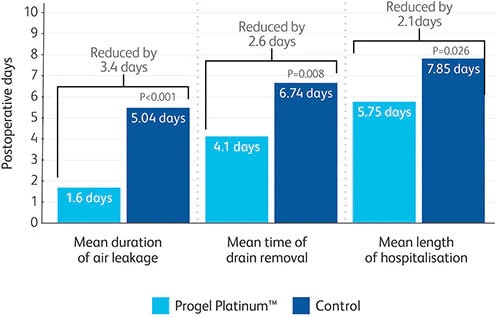
Clinically proven to seal air leaks and reduce the length of stay by an average of 2.1 days.8
Progel Platinum™ Surgical Sealant is a specialised sealant designed with a unique combination of strength, flexibility and adherence, clinically proven to seal leaks and reduce length of hospital stay by 2.1 days.8