Hospitals using the BD MaxPlus™ needle-free connector had lower unadjusted central line-associated bloodstream infection (CLABSI) rates, as well as lower standardised infection ratios, compared to hospitals not using the MaxPlus technology.*1
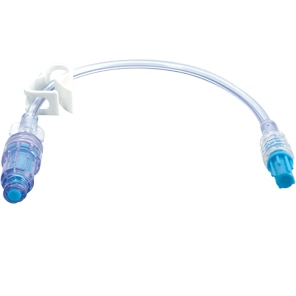
BD MaxPlus™ Needle-Free Connector
Choose the only needle-free connector with an FDA-cleared label statement demonstrating a reduction in CLABSI

- Overview
- EIFU & Resources

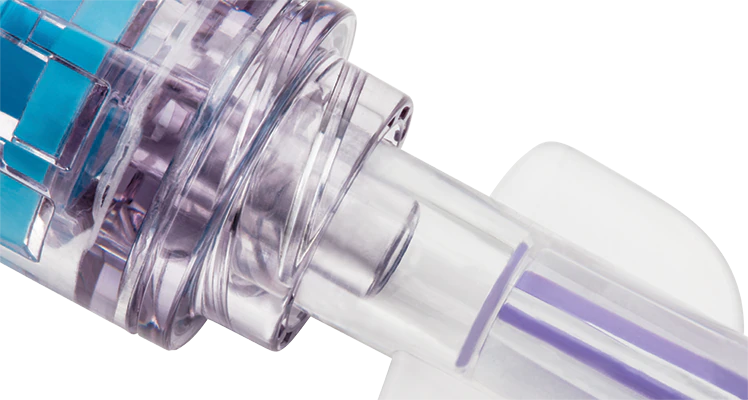
Solid sealed surface
The solid sealed surface withstands 7 days/200 activations.

Clear housing
The clear design shows the complete fluid pathway, enabling optimal visibility when flushing.

No interstitial space
Zero interstitial space between the components limits opportunities for microbial ingress.
Zero-reflux action
Zero-reflux feature upon disconnection can help reduce catheter occlusions and catheter occlusions
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
* 2013 CMS hospital compare data from 3,074 hospitals, accounting for nearly 11,000 CLABSIs associated with nearly 10 million catheter days.
- Tabak YP, Johannes RS, Sun X, Crosby CT, Jarvis WR. Innovative use of existing public and private data sources for postmarket surveillance of central line-associated bloodstream infections associated with intravenous needleless connectors. J Infus Nurs. 2016;39(5):328-335.
BD-35577 (08/2021)

.png)