Indication for Use
The Aspirex™ Mechanical Aspiration Thrombectomy System is indicated for the removal of acute emboli and thrombi from vessels in the peripheral venous system.
Contraindications
Not for use in the vessels of the cardiac, pulmonary, coronary and neurovasculature.
Warnings
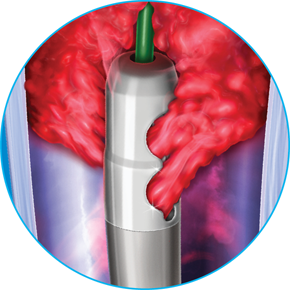
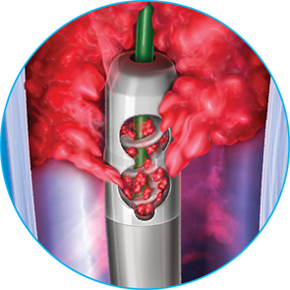
Before using the Aspirex™ Endovascular System and its components, the user must be entirely familiar with the user manuals of the Aspirex™ Drive System and Aspirex™ rotational catheters; Only use sheaths that are highly resistant to kinking. If used incorrectly, Aspirex™ Mechanical Aspiration Thrombectomy System and/or the guidewire used can cause vessel perforation; Insert and operate the catheter over the supplied guidewire of the appropriate length only. During the procedure, unforeseen complications of technical or medical origin may make it necessary to carry out unplanned, emergency additional measures, such as, but not limited to, administration of thrombolytic agents or surgical intervention; The products are for single use and must not be resterilized; Do not use the products after the expiration date; Appropriate testing of the patient’s coagulation status is mandatory. Aspirex™ Mechanical Aspiration Thrombectomy System may only be used in the indicated diameters of target vessels. The catheter must always be guided via the guidewire, which has been correctly positioned according to the instructions for use. Make sure that the flexible tip of the guidewire is placed as distal as possible to the occluded segment to prevent the flexible tip from being aspirated into the catheter head. The guidewire must lie inside the lumen throughout its course from the introducing sheath to its flexible tip. Monitor the correct position of the guidewire throughout the entire process of catheter use. The catheter must never be kinked at any stage. At no point should the catheter ever be exposed to pressure that is sufficient to compress the tube so that it is pressed against the rotating helix. The catheter lumen must be filled with liquid (heparinised isotonic saline or blood) at all times throughout catheter use in the patient. If resistance is experienced, pull the catheter back a little way into the open(ed) segment with the motor continuing to run so that the ablated material can be processed and carried away. Advancing the catheter too quickly increases the risk of this advancement mobilising more material than can be aspirated and carried away, which can cause distal embolization
Cautions
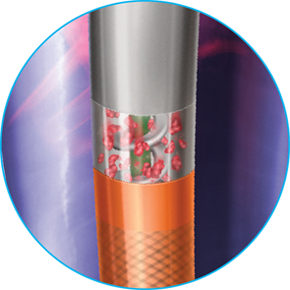
The internal lumen of the introducer sheath must at least correspond to the external diameter of the catheter. At all times monitor the quantity of blood transported into the collecting bag. Effective anticoagulants at a suitable dose have to be administered before the patient is treated with the Straub Endovascular System in order to achieve an activated clotting time (ACT) > 250 seconds or equivalent values according to other measuring techniques, throughout use of the catheter. If used correctly, embolizations caused by material detached by the catheter head are very rare. Ensure that the catheter lumen is completely filled with solution when the motor is running. The wire adapter must be in the working position (knob pulled out) during use of the catheter. If there is unlikely to be enough natural flow of blood to the catheter head, the supply of liquid to the catheter head can be guaranteed by providing additional appropriate liquid, such as isotonic saline, via a suitable access, such as the side-port of the introducer sheath being used; If the LEDs go out or the alarm is audible, safe functioning of the catheter is no longer guaranteed. Blood and thrombus fragments in the catheter lumen might clot if the helix has stopped. Therefore, if catheter use is interrupted, the catheter must be rinsed immediately in heparinised isotonic saline
Precautions
The catheter sets do not contain any parts that need to be maintained or serviced by the end-user. Do not repair or change the configuration of the product. An annual service is recommended for the Straub Medical Drive System (see Straub Medical Drive System user manual)
Potential Adverse Effects
Embolisms, especially distal thromboembolisms; pulmonary embolisms of all degrees of severity; thromboses, especially recurrent thromboses; re-occlusion; vessel wall injury or valve damage; vessel dissection / perforation / rupture; perforation as a result of mural calcium being torn out of the vessel wall; arteriovenous fistula / pseudo-aneurysm; hematoma, bleeding, hemorrhage; organ perforation; implants such as stents / stent grafts / bypass grafts getting damaged, caught or dislodged; disruption of the catheter and/or guidewire: debris remaining in the body; allergic reactions to catheter material; death; infections or necrosis at the puncture site; allergic reactions; catheter-induced sepsis
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings, and precautions