BD PhaSeal™ Optima system
The next-generation Closed-System Drug-Transfer Device (CSTD) from BD that advances hazardous drug protection

- Overview
- Innovation
- Applications
- Product & Accessories
- eIFU & Resources
The BD PhaSeal™ System pioneered the category of closed-system drug transfer devices (CSTDs) to help protect the pharmacists and clinicians who prepare and administer hazardous drugs. 20 years later, we turned to healthcare professionals like you for feedback and guidance to optimize its every component. The result is the BD PhaSeal Optima System—a next-generation, user-tested CSTD solution that advances hazardous drug safety.
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
With design innovations in every component
Every component of the BD PhaSeal Optima System is designed with no inlets or air exchange for airtight hazardous drug transfers. Click on each hot spot below to see how we have also optimized ease of use, ergonomics and performance.
Every component of the BD PhaSeal Optima System is designed with no inlets or air exchange for airtight hazardous drug transfers. Click on each hot spot below to see how we have also optimized ease of use, ergonomics and performance.

- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
Compatible
with ISO-compliant syringes and IV sets
Power grip and straight motion to connect
Aligned with the recommendations of the National Institute for Occupational Safety and Health (NIOSH)1
No alignment or orientation needed for connection
Ease of use helps to minimize learning curve
Proprietary protector
Designed to maximize drug extraction for each vial
Injector
Designed to prevent needle exposure and accidental needlesticks
Component design
Optimized for clinician comfort
Intuitive straight-push connection
Connects in one step, helping workflow integration
Locking Injector
Includes a locking feature that locks securely to the mated Luer device.
Notes
* Within an ISO Class V environment following aseptic technique
** 2015
^ The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending or superseding a manufacturer's labeling recommendations for the storage and expiration dating of the drug vial. Refer to drug manufacturer's recommendations and USP compounding guidelines for shelf life and sterility information.
References
- Centers for Disease Control and Prevention (CDC). Easy Ergonomics: A Guide to Selecting Non-Powered Hand Tools. https://www.cdc.gov/niosh/docs/2004-164/pdfs/2004-164.pdf. Published August 18, 2004. Accessed September 2, 2020.
- BD Engineering study: Evaluation of PhaSeal Optima residual drug loss performance testing. 2017.
- BD Engineering study: Evaluation of PhaSeal Optima residual drug loss performance testing. 2018.
- Gilbar PJ, Chambers CR, Gilbar EC. Opportunities to significantly reduce expenditure associated with cancer drugs. Future Oncol.2017;13(15):1311-1322.
- McMichael DM, Jefferson DM, Carey ET, et al. Utility of the PhaSeal closed system drug transfer device. Am J Pharm Benefits.2011;3(1):9-16.
- Bendeka [prescribing information], North Wales, Pennsylvania. Teva Pharmaceuticals USA, Inc. 2018.
- Doxil [prescribing information], Bedford, Ohio. ALZA Corporation. 2016.
- Herceptin [prescribing information], South San Francisco, California. Genentech, Inc. 2017.
- Valstar [prescribing information], Chadds Ford, Pennsylvania. Endo Pharmaceuticals, Inc. 2017
- April 2018 ASP pricing file. CMS website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2018ASPFiles.html. Accessed June 8, 2018.
BD-17517 (06/24)
Designed to address your economic concerns
Ease of use
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
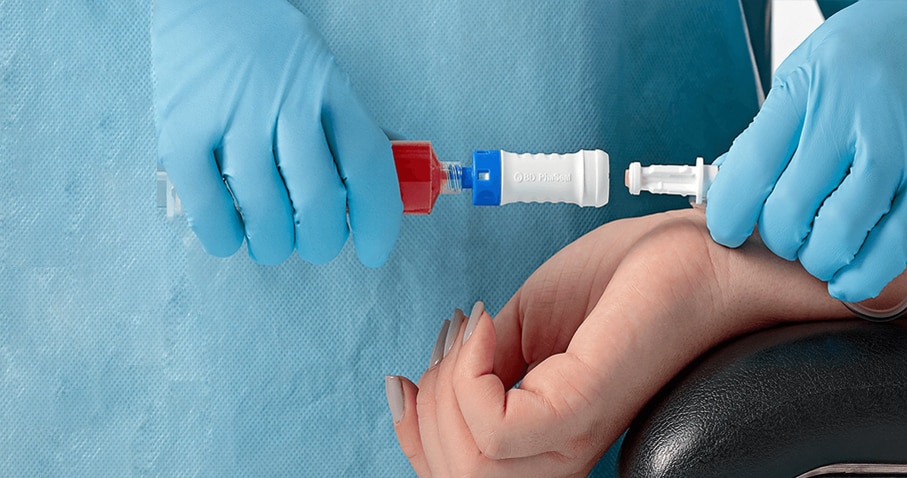
The BD PhaSeal™ Optima System is easy to use, and connects with one step
The BD PhaSeal™ Optima System replaces the push-turn-push connection mechanism of the BD PhaSeal™ System with a one-step straight-push mechanism, making it easier for pharmacists and clinicians to use.
Minimize learning curve
Designed to help minimize the learning curve with:
- Intuitive one-step straight-push connection
- No alignment or orientation needed to connect
Let our trained clinical specialists help you efficiently integrate the BD PhaSeal™ Optima System into the workflow of your facility.
Ergonomics
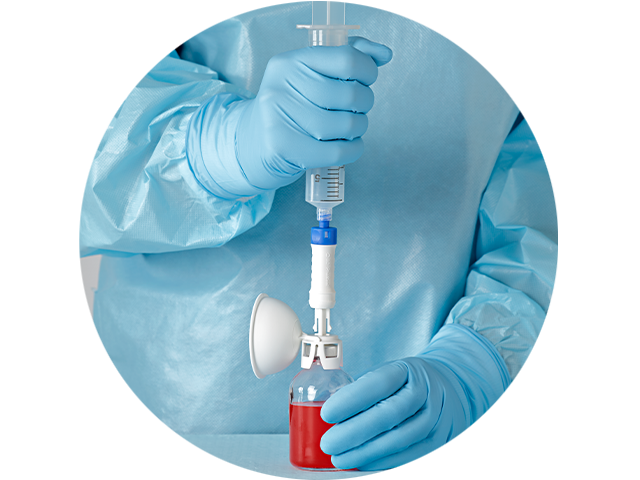
Offer your pharmacists and clinicians an ergonomic CSTD solution
We conducted head-to-head comparisons and asked both longstanding CSTD users and traditional technique users how we could improve our design. The feedback and validation we received guided us to optimize every component of the BD PhaSeal™ Optima System for user comfort.

Align with NIOSH recommendations1
- Uses a power grip and straight motion to connect and disconnect
- Aligns with NIOSH recommendations to avoid pinch grip for hand-held tools1
Our trained clinical specialists can also help train your pharmacists and clinicians in best practices for preparation and administration using the BD PhaSeal™ Optima System.
Performance
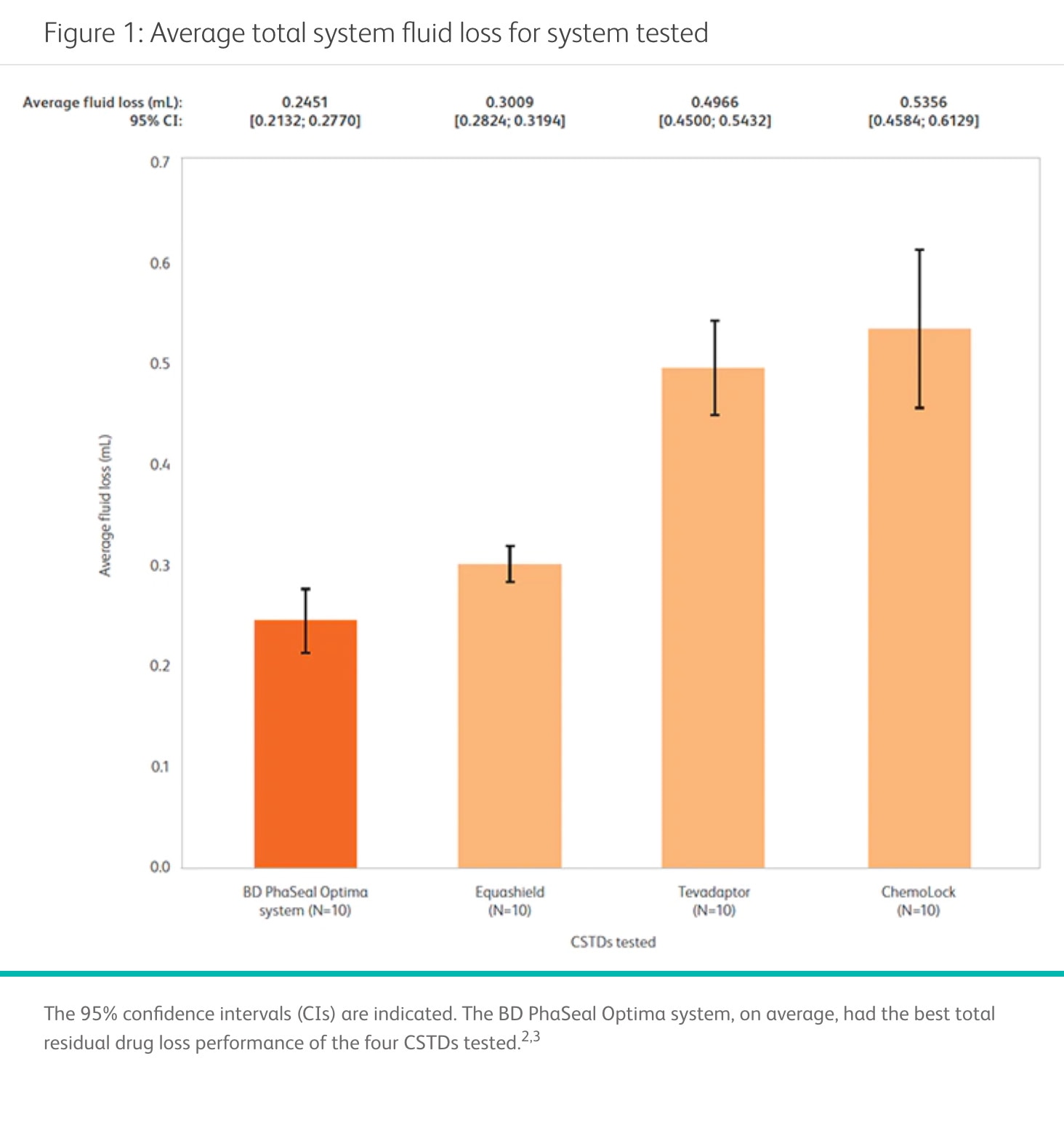
The BD PhaSeal™ Optima System can help you maximize drug extraction for each vial
In a performance comparison, the BD PhaSeal™ Optima System demonstrated the lowest residual fluid loss of all four CSTDs tested.2,3* Minimizing residual fluid loss with such a CSTD may translate into a reduction in drug waste that can help optimize preparation of HDs and increase cost savings for your facility. 4,5
*Bench test results may not be indicative of clinical results.

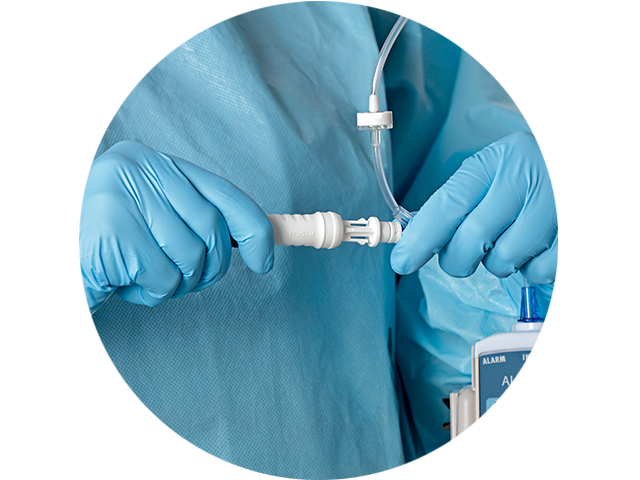
Designed to maximize performance
- Prevents microbial ingress and may maintain drug vial sterility for up to 168 hours and 10 penetrations**^
- Meets the requirements of the NIOSH IPA draft CSTD-test protocol, a robust test protocol applicable to barrier-type CSTDs**
- Optimized spike-fluid channel and secure vial attachment
- Engineered to common International Organization for Standardization (ISO) standards
- Compatible and connects with ISO-compliant syringes, IV sets, luer fittings and standard-size drug vials
We can share the 'Minimizing residual drug loss' and 'Assessment of microbial ingress' whitepapers with you, and help you calculate potential drug savings for your facility.
*Within an ISO Class V environment following aseptic technique. The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending or superseding a manufacturer's labeling recommendations for the storage and expiration dating of the drug vial. Refer to drug manufacturer's recommendations and USP compounding guidelines for shelf life and sterility information.
Safety
Designed to reduce the risk of surface and environmental contamination of hazardous drugs
The BD PhaSeal™ Optima System may help improve healthcare worker safety by reducing the risk of surface contamination to hazardous drugs. The BD PhaSeal™ Optima System incorporates the double membranes and vapor capture mechanisms pioneered by the BD PhaSeal™ System, which has been demonstrated to reduce the risk of hazardous drug exposure.

Secure your Luer lock connections with the option of the N40-O locking injector
- Locking feature that locks securely to the mated Luer device
- Spinning collar enables rotation of syringe to view scale markings and prevent tangling of IV lines
- May reduce drug leaks, large spills and waste
- Helps prevent leaks in transport to clinical settings
- Helps reduce risk of delays in treatment that could lead to under-dosing and patient dissatisfaction

Leakproof and airtight by design
- Spike design prevents vapor leakage when puncturing a vial
- Self-sealing membranes on all components
- Airtight drug transfers in preparation and administration
- Leakproof for both short- and long-term connections
Let our trained clinical specialists help you seamlessly upgrade your facility to the BD PhaSeal Optima System.
Notes
* Within an ISO Class V environment following aseptic technique
** 2015
^ The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending or superseding a manufacturer's labeling recommendations for the storage and expiration dating of the drug vial. Refer to drug manufacturer's recommendations and USP compounding guidelines for shelf life and sterility information.
References
- Centers for Disease Control and Prevention (CDC). Easy Ergonomics: A Guide to Selecting Non-Powered Hand Tools. https://www.cdc.gov/niosh/docs/2004-164/pdfs/2004-164.pdf. Published August 18, 2004. Accessed September 2, 2020.
- BD Engineering study: Evaluation of PhaSeal Optima residual drug loss performance testing. 2017.
- BD Engineering study: Evaluation of PhaSeal Optima residual drug loss performance testing. 2018.
- Gilbar PJ, Chambers CR, Gilbar EC. Opportunities to significantly reduce expenditure associated with cancer drugs. Future Oncol.2017;13(15):1311-1322.
- McMichael DM, Jefferson DM, Carey ET, et al. Utility of the PhaSeal closed system drug transfer device. Am J Pharm Benefits.2011;3(1):9-16.
- Bendeka [prescribing information], North Wales, Pennsylvania. Teva Pharmaceuticals USA, Inc. 2018.
- Doxil [prescribing information], Bedford, Ohio. ALZA Corporation. 2016.
- Herceptin [prescribing information], South San Francisco, California. Genentech, Inc. 2017.
- Valstar [prescribing information], Chadds Ford, Pennsylvania. Endo Pharmaceuticals, Inc. 2017
- April 2018 ASP pricing file. CMS website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2018ASPFiles.html. Accessed June 8, 2018.
BD-17517 (06/24)
Easy to use in the preparation and administration of hazardous drugs

See how pharmacists and clinicians can implement the airtight and leakproof BD PhaSeal™ Optima System.
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD PhaSeal™ Optima System instructional video for preparation
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD PhaSeal™ Optima System instructional video for administration

Notes
* Within an ISO Class V environment following aseptic technique
** 2015
^ The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending or superseding a manufacturer's labeling recommendations for the storage and expiration dating of the drug vial. Refer to drug manufacturer's recommendations and USP compounding guidelines for shelf life and sterility information.
References
- Centers for Disease Control and Prevention (CDC). Easy Ergonomics: A Guide to Selecting Non-Powered Hand Tools. https://www.cdc.gov/niosh/docs/2004-164/pdfs/2004-164.pdf. Published August 18, 2004. Accessed September 2, 2020.
- BD Engineering study: Evaluation of PhaSeal Optima residual drug loss performance testing. 2017.
- BD Engineering study: Evaluation of PhaSeal Optima residual drug loss performance testing. 2018.
- Gilbar PJ, Chambers CR, Gilbar EC. Opportunities to significantly reduce expenditure associated with cancer drugs. Future Oncol.2017;13(15):1311-1322.
- McMichael DM, Jefferson DM, Carey ET, et al. Utility of the PhaSeal closed system drug transfer device. Am J Pharm Benefits.2011;3(1):9-16.
- Bendeka [prescribing information], North Wales, Pennsylvania. Teva Pharmaceuticals USA, Inc. 2018.
- Doxil [prescribing information], Bedford, Ohio. ALZA Corporation. 2016.
- Herceptin [prescribing information], South San Francisco, California. Genentech, Inc. 2017.
- Valstar [prescribing information], Chadds Ford, Pennsylvania. Endo Pharmaceuticals, Inc. 2017
- April 2018 ASP pricing file. CMS website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2018ASPFiles.html. Accessed June 8, 2018.
BD-17517 (06/24)
-
Protect clinicians and patients from exposure to hazardous drugs with a closed system
-

Get results in less than 10 minutes with the first and only rapid hazardous drug* detection system
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.




