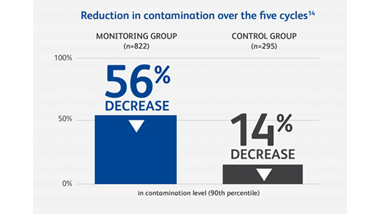
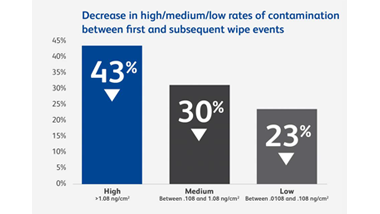
Anyone who handles hazardous drugs (HDs) during transportation, preparation, administration or waste disposal may be at risk. A Canadian study of six hospitals found that frequently contacted surfaces at every stage of the hospital medication system had measurable levels of antineoplastic drug contamination.9
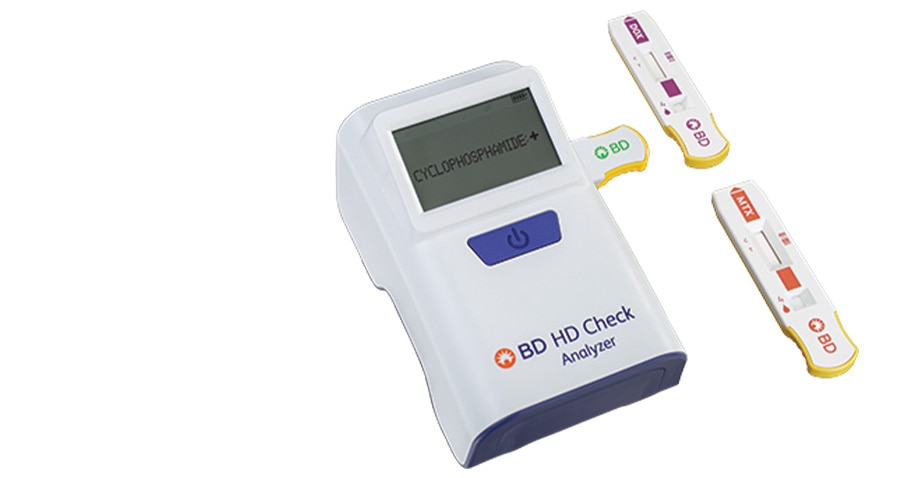
The revolutionary BD® HD Check System has a convenient handheld design that enables testing for select HDs at any location. It provides reliable, easy-to-read results in less than 10 minutes, so you can take immediate corrective action and prevent HD residue from spreading.