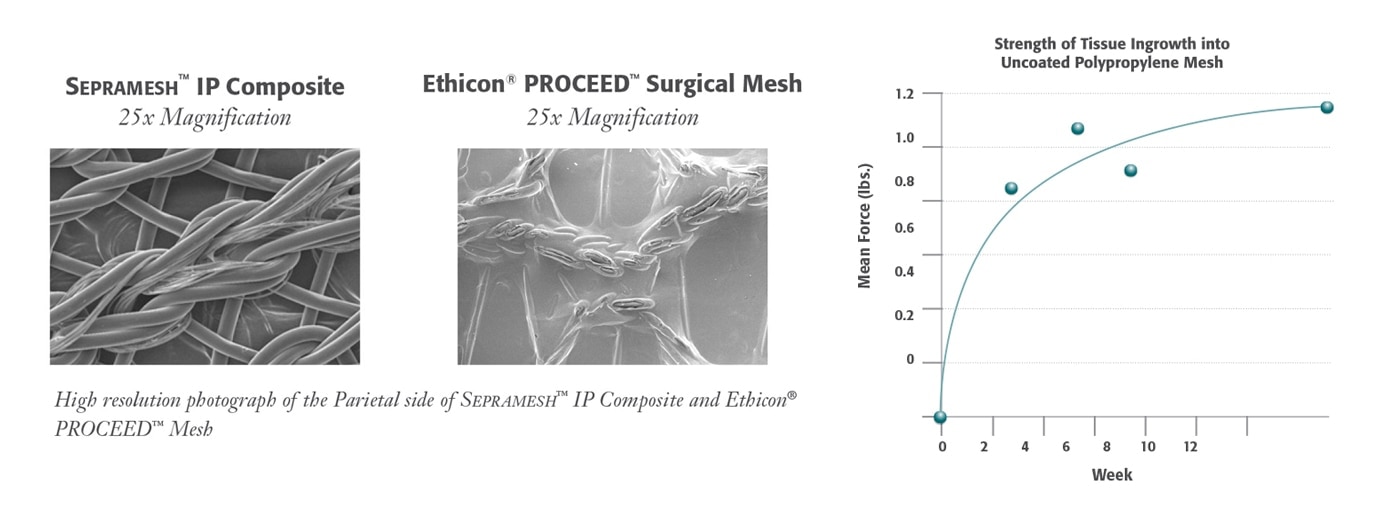
* Preclinical data on file. Results may not correlate to performance in humans.
1 Sasse KC, Lim DCL, Brandt J, “Long-term Durability and Comfort of Laparoscopic Ventral Hernia Repair”. Journal of the Society of Laparoendoscopic Surgeon, 2012 July-September, 16(3): 380-386
INDICATIONS
Sepramesh™ IP Bioresorbable Coating / Permanent Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRAINDICATIONS
1. Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
2. Do not use Sepramesh™ IP Bioresorbable Coating/Permanent Mesh in infants or children, whereby future growth will be compromised by use of such mesh material.
3. Do not use Sepramesh™ IP Bioresorbable Coating/Permanent Mesh for the reconstruction of cardiovascular defects.
WARNINGS
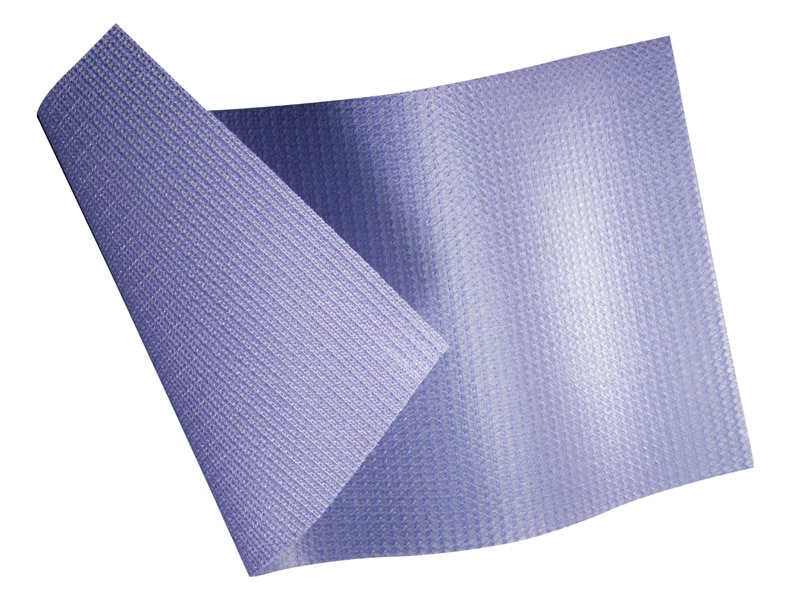
Ensure proper orientation; the coating side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the prosthesis is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-14802