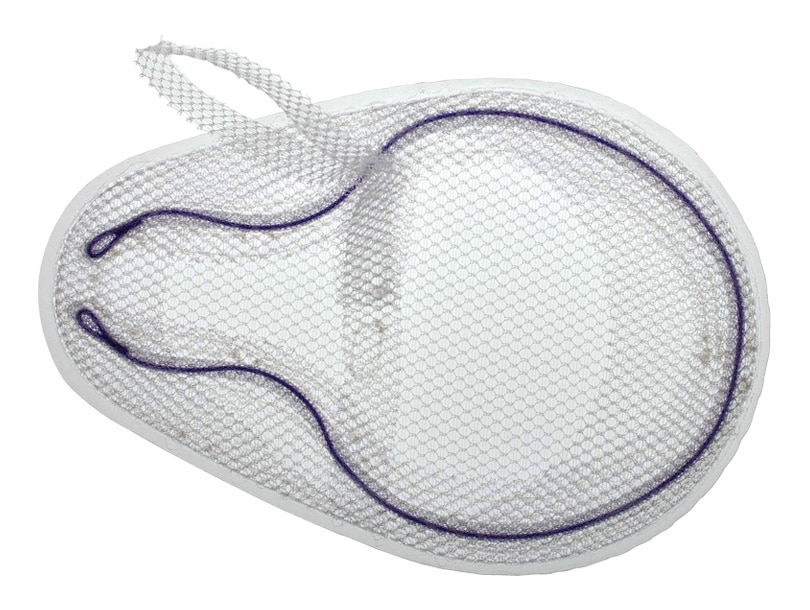
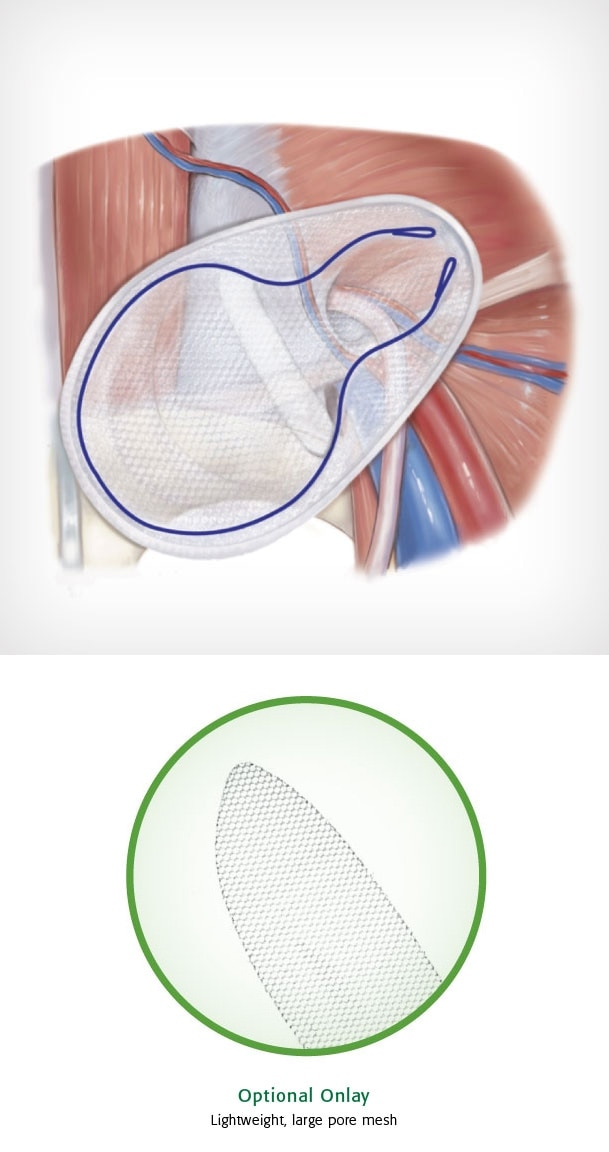
Bard© Modified OnFlex ™ Mesh was specifically designed to fit the inguinal anatomy during preperitoneal placement. It offers extended medial and inferior coverage for direct and femoral hernia spaces.
- Overview
- Products & Accessories
- EIFU & Resources
- Reduces the amount of foreign material implanted
- Allows good tissue ingrowth1*
- Results in a more flexible and compliant scar formation1
- Medial and lateral pockets aid in proper placement
Absorbable Sobraflex Memory Technology

1. Brown C, Finch J. Which mesh for hernia repair? Annals of The Royal College of Surgeons of England 2010;92(4):272-278.
* Observed in preclinical model, which may not correlate to performance in humans.
Indications
The Modified Onflex™ Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, such as in the repair of inguinal hernias.
Contraindications
Use of this device is contraindicated for infants, children, or pregnant women, whereby future growth will be compromised by use of such mesh material. Literature reports that there is a possibility for adhesion formation when polypropylene is placed in direct contact with the bowel or viscera.
Warnings
The use of any synthetic mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh and is not recommended. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. Unresolved infection may require removal of the mesh.
Do not cut or reshape the Modified Onflex™ Mesh, except for the positioning strap, as this could affect its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament
Excess positioning strap material above the fixation point must be cut off at the level of the fascia and discarded to eliminate excess material from remaining in the body.
Precautions
Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament.
Adverse Reactions
Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, extrusion, erosion, migration, fistula formation and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged, additional complications may include, but are not limited to, bowel or skin perforation and infection.
1. Brown C, Finch J. Which mesh for hernia repair? Annals of The Royal College of Surgeons of England 2010;92(4):272-278.
* Observed in preclinical model, which may not correlate to performance in humans.
Indications
The Modified Onflex™ Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, such as in the repair of inguinal hernias.
Contraindications
Use of this device is contraindicated for infants, children, or pregnant women, whereby future growth will be compromised by use of such mesh material. Literature reports that there is a possibility for adhesion formation when polypropylene is placed in direct contact with the bowel or viscera.
Warnings
The use of any synthetic mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh and is not recommended. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. Unresolved infection may require removal of the mesh.
Do not cut or reshape the Modified Onflex™ Mesh, except for the positioning strap, as this could affect its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament
Excess positioning strap material above the fixation point must be cut off at the level of the fascia and discarded to eliminate excess material from remaining in the body.
Precautions
Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament.
Adverse Reactions
Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, extrusion, erosion, migration, fistula formation and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged, additional complications may include, but are not limited to, bowel or skin perforation and infection.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Training resources to help improve your clinical practices as part of our goal of advancing the world of health.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
1. Brown C, Finch J. Which mesh for hernia repair? Annals of The Royal College of Surgeons of England 2010;92(4):272-278.
* Observed in preclinical model, which may not correlate to performance in humans.
Indications
The Modified Onflex™ Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, such as in the repair of inguinal hernias.
Contraindications
Use of this device is contraindicated for infants, children, or pregnant women, whereby future growth will be compromised by use of such mesh material. Literature reports that there is a possibility for adhesion formation when polypropylene is placed in direct contact with the bowel or viscera.
Warnings
The use of any synthetic mesh or patch in a contaminated or infected wound can lead to fistula formation and/or extrusion of the mesh and is not recommended. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. Unresolved infection may require removal of the mesh.
Do not cut or reshape the Modified Onflex™ Mesh, except for the positioning strap, as this could affect its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament
Excess positioning strap material above the fixation point must be cut off at the level of the fascia and discarded to eliminate excess material from remaining in the body.
Precautions
Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament.
Adverse Reactions
Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, extrusion, erosion, migration, fistula formation and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged, additional complications may include, but are not limited to, bowel or skin perforation and infection.