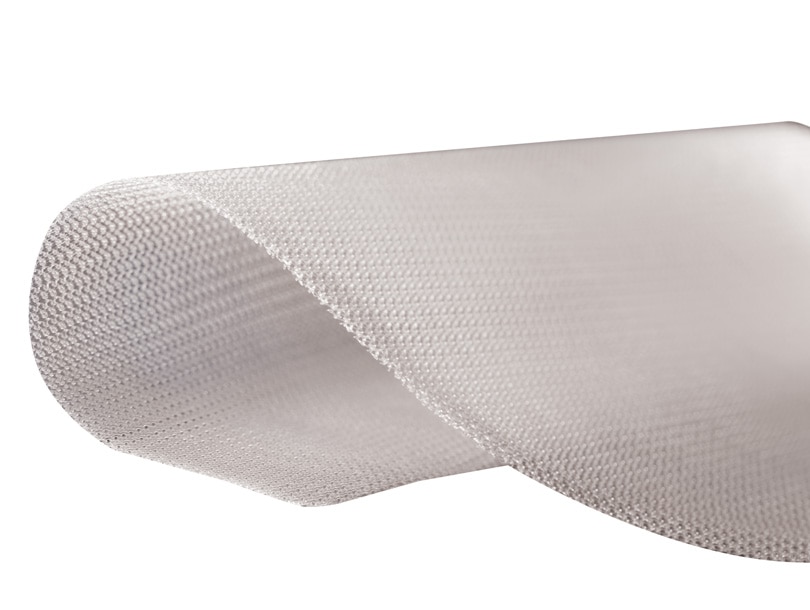
Renfort de paroi Phasix™ ST
Prothèse entièrement biorésorbable intégrant la technologie éprouvée Sepra®

- Présentation
- Instructions d'utilisation et ressources
Designed to enable functional tissue remodeling for a strong repair
Phasix™ ST Mesh combines two market-leading technologies into one product: monofilament resorbable Phasix™ Mesh and a proven hydrogel barrier based on Sepra® technology. While the monofilament mesh supports functional healing and a strong repair, the hydrogel barrier minimizes tissue attachment to the visceral side of the mesh for intraabdominal placement.1
La prochaine phase de la réparation de hernie
Réparations.1
La structure monofilament assure une intégration rapide et une réparation solide.1
Remodelages.1
L’intégration vasculaire et l’incorporation se poursuivent, avec une abondance de collagène mature à 52 semaines. La charge est transférée vers le tissu natif au fil du temps. 1
Restauration.1
Au fur et à mesure que la maille Phasix™ est remodelée, elle est remplacée par du tissu fonctionnel, pour entraîner finalement une solide réparation au bout d’un an.1
Structure du matériau1
Il a été déterminé que les modèles de mailles monofilament offrent une biocompatibilité supérieure et présentent moins de risques d’adhérence et de colonisation bactériennes.1
References
Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
DeMeester, Steven R, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
Phasix™ ST Mesh is indicated for use in the reinforcement of abdominal soft tissue, where weakness exists, in ventral and hiatal hernia repair procedures.
Contraindications
Because Phasix™ ST Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Mesh manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this mesh in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
Ensure proper orientation; the coated side of the mesh should be oriented against the bowel or sensitive organs. Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
The safety and effectiveness of Phasix™ ST Mesh in bridging repairs has not been evaluated or established.
The safety and effectiveness of Phasix™ ST Mesh in laparoscopic/robotic ventral hernia repair procedures has not been evaluated or established.
The use of any mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh and it is not recommended.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require the removal of the mesh.
To prevent recurrences when repairing hernias, mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
For hiatal hernia repair, the use of Phasix™ ST Mesh circumferentially around the esophagus is not recommended.
For hiatal hernia repair, the use of Phasix™ ST Mesh to bridge the hiatus is not recommended.
The safety and effectiveness of Phasix™ ST Mesh in the following applications has not been evaluated or established: a. Pregnant or breastfeeding women b. Pediatric use.
Product should be used once exterior foil pouch has been opened. Do not store for later use.
Unused portions of the mesh should be discarded. If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard mesh with care to prevent risk of transmission of viral and other infections.
This mesh is designed for single use only. Reuse, resterilization, reprocessing and/or repackaging of any portion of the Phasix™ ST Mesh may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness, or death of the patient or end user.
This mesh is supplied sterile. Prior to use, carefully examine package and product to verify neither is damaged and that all seals are intact. Do not use if the foil pouch or package is damaged or open, or if the center of the temperature indicator on the foil pouch is black.
This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
This mesh is not for the use of treatment of stress urinary incontinence.
This mesh is not for use of repair of neural and cardiovascular tissue.
Phasix™ ST Mesh has not been studied for use in breast reconstructive surgeries.
Precautions.
Please read all instructions prior to use.
Only physicians qualified in the appropriate surgical techniques should use this mesh. Users should be familiar with strength and mesh size requirements. Improper selection, placement, positioning and fixation of the mesh can cause subsequent undesirable results.
The safety and effectiveness of the mesh has not been evaluated in the presence of malignancies in the abdominopelvic cavity.
The safety and effectiveness of Phasix™ ST Mesh in the proximity of existing or excised cancer has not been established.
Adverse Reactions.
In preclinical testing, Phasix™ ST Mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, allergic reaction, hemorrhage, extrusion, erosion, migration, fistula formation, and recurrence of the hernia or soft tissue defect. Possible complications in hiatal hernia repair may include esophageal erosion and dysphagia related to crural fibrosis. Please consult package insert for more detailed safety information and instructions for use.
References
Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
DeMeester, Steven R, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020
Disclaimers
Not all products, services, claims or features of products may be available or valid in your local area. Please check with your local BD representative.
Please consult product labels and instructions for use for indications, contradictions, hazards, warnings, and precautions.
Indications
Phasix™ ST Mesh is indicated for use in the reinforcement of abdominal soft tissue, where weakness exists, in ventral and hiatal hernia repair procedures.
Contraindications
Because Phasix™ ST Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Mesh manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this mesh in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
Ensure proper orientation; the coated side of the mesh should be oriented against the bowel or sensitive organs. Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
The safety and effectiveness of Phasix™ ST Mesh in bridging repairs has not been evaluated or established.
The safety and effectiveness of Phasix™ ST Mesh in laparoscopic/robotic ventral hernia repair procedures has not been evaluated or established.
The use of any mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh and it is not recommended.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require the removal of the mesh.
To prevent recurrences when repairing hernias, mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
For hiatal hernia repair, the use of Phasix™ ST Mesh circumferentially around the esophagus is not recommended.
For hiatal hernia repair, the use of Phasix™ ST Mesh to bridge the hiatus is not recommended.
The safety and effectiveness of Phasix™ ST Mesh in the following applications has not been evaluated or established: a. Pregnant or breastfeeding women b. Pediatric use.
Product should be used once exterior foil pouch has been opened. Do not store for later use.
Unused portions of the mesh should be discarded. If unused mesh has been in contact with instruments or supplies used on a patient or contaminated with body fluids, discard mesh with care to prevent risk of transmission of viral and other infections.
This mesh is designed for single use only. Reuse, resterilization, reprocessing and/or repackaging of any portion of the Phasix™ ST Mesh may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the mesh and may lead to mesh failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the mesh and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the mesh may lead to injury, illness, or death of the patient or end user.
This mesh is supplied sterile. Prior to use, carefully examine package and product to verify neither is damaged and that all seals are intact. Do not use if the foil pouch or package is damaged or open, or if the center of the temperature indicator on the foil pouch is black.
This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach.
This mesh is not for the use of treatment of stress urinary incontinence.
This mesh is not for use of repair of neural and cardiovascular tissue.
Phasix™ ST Mesh has not been studied for use in breast reconstructive surgeries.
Precautions.
Please read all instructions prior to use.
Only physicians qualified in the appropriate surgical techniques should use this mesh. Users should be familiar with strength and mesh size requirements. Improper selection, placement, positioning and fixation of the mesh can cause subsequent undesirable results.
The safety and effectiveness of the mesh has not been evaluated in the presence of malignancies in the abdominopelvic cavity.
The safety and effectiveness of Phasix™ ST Mesh in the proximity of existing or excised cancer has not been established.
Adverse Reactions.
In preclinical testing, Phasix™ ST Mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, allergic reaction, hemorrhage, extrusion, erosion, migration, fistula formation, and recurrence of the hernia or soft tissue defect. Possible complications in hiatal hernia repair may include esophageal erosion and dysphagia related to crural fibrosis. Please consult package insert for more detailed safety information and instructions for use.
Le filet Phasix™ ST est indiqué pour le renforcement des tissus mous abdominaux, dans les cas de hernies ventrales et hiatales. Dispositif médical de classe III MDR (EU) 2017/745, CE 2797. Fabricant : Davol inc. Pour un bon usage se référer à la notice d'utilisation. Edition Juillet 2023
Références
- Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
- DeMeester, Steven R, et al. L’association d’une technique chirurgicale et du renforcement par maille biorésorbable de la réparation crurale permet d’obtenir un faible taux de récidive précoce des hernies lors de la réparation par laparoscopie d’une hernie para-œsophagienne. J Gastrointest Surg. 2020
BD-119798