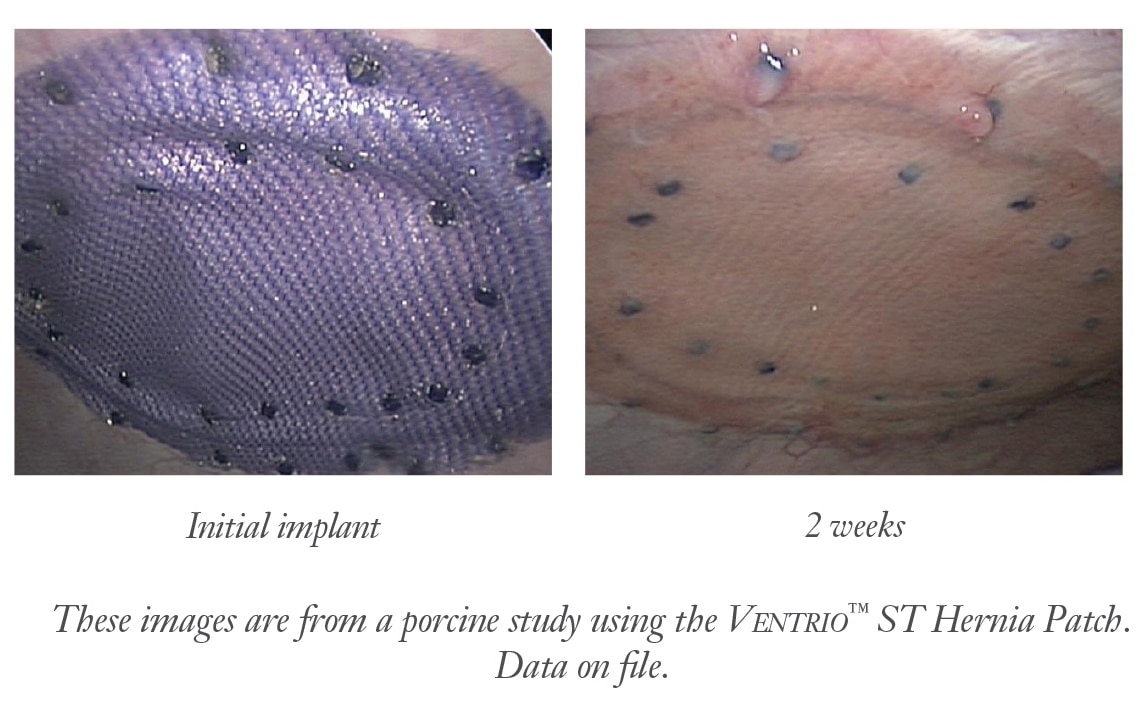
1 Preclinical data on file at C. R. Bard. Results may not correlate to performance in humans.
2 Iannitti, D. et. al. “Technique and Outcomes of Abdominal Incisional Hernia Repair Using a Synthetic Composite Mesh: A Report of 455 cases.” Journal of the American College of Surgeons. 2008 Jan; 206 (1):83-8.
3 Majercik, S. et al. “Strength in tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endosc (2006) 20: 1671-1674.
INDICATIONS
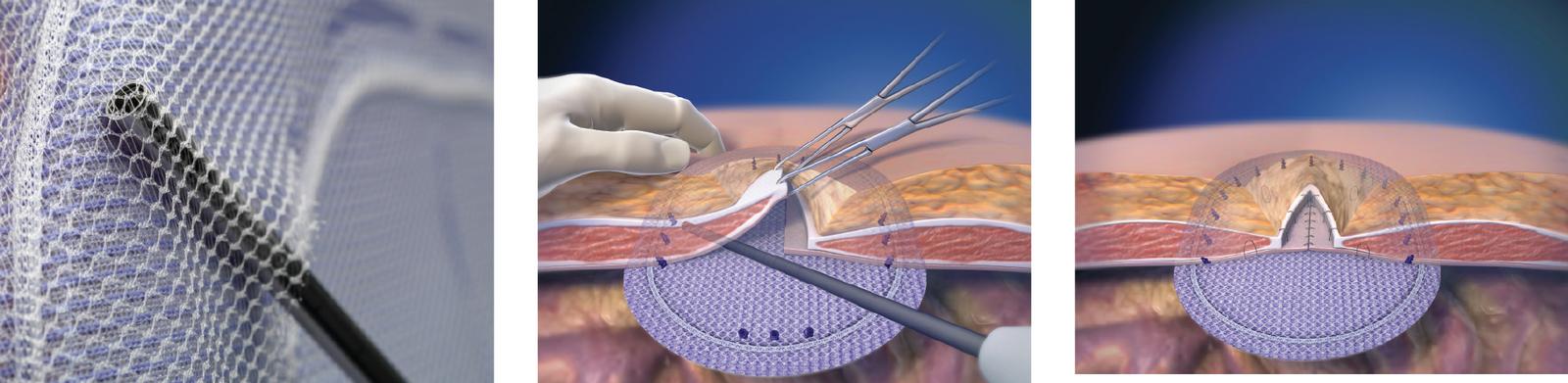
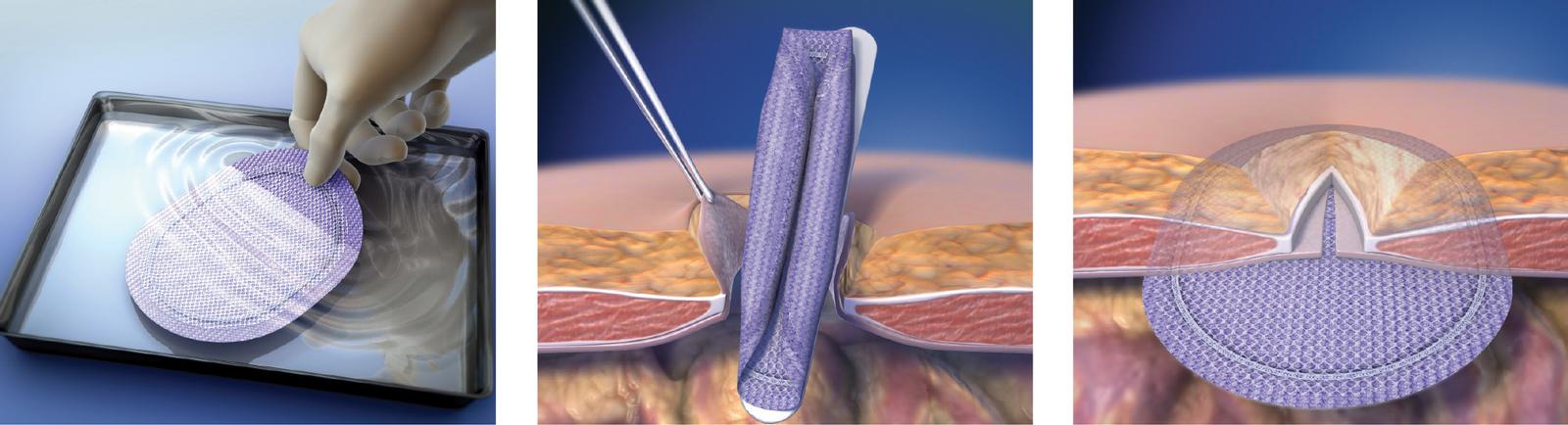
Ventrio™ ST Hernia Patch is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair of ventral, incisional, and umbilical hernias.
CONTRAINDICATIONS
Do not use the Ventrio™ ST Hernia Patch in infants or children, whereby future growth will be compromised by the use of such mesh material. Do not use the Ventrio™ ST Hernia Patch for the reconstruction of cardiovascular defects. Literature reports that there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
Do not cut or reshape the Ventrio™ ST Hernia Patch, as this could impact its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament during insertion or fixation. If the SorbaFlex™ PDO monofilament is cut or damaged, additional complications may include bowel or skin perforation and infection. Follow proper folding techniques for all patches as described in the Instructions for Use as other folding techniques may compromise the SorbaFlex™ PDO monofilament. Ensure proper orientation; the bioresorbable coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene mesh side against the bowel. There may be a possibility for adhesion formation when the mesh is placed in direct contact with the bowel or viscera.
This mesh is not for the use of repair of pelvic organ prolapse via transvaginal approach. This mesh is not for the use of treatment of stress urinary incontinence.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.