BD Nexiva™ Closed IV Catheter System is not just a catheter. BD Nexiva™ Closed IV Catheter System is an all-in-one PIVC, combining four individual devices in one: IV catheter, extension set, stabilization device, and needle-free connector/s. It is clinically demonstrated to have longer dwell times – dwelling up to 144 hours – and reduced complications versus an open system.*
*with a PTFE catheter

- Overview
- Best Practices
- Products & Accessories
- EIFU & Resources

BD Vialon™ catheter material
Proprietary BD Vialon™ Catheter Material softens, enabling longer dwell time and reducing the chance of phlebitis up to 69%.
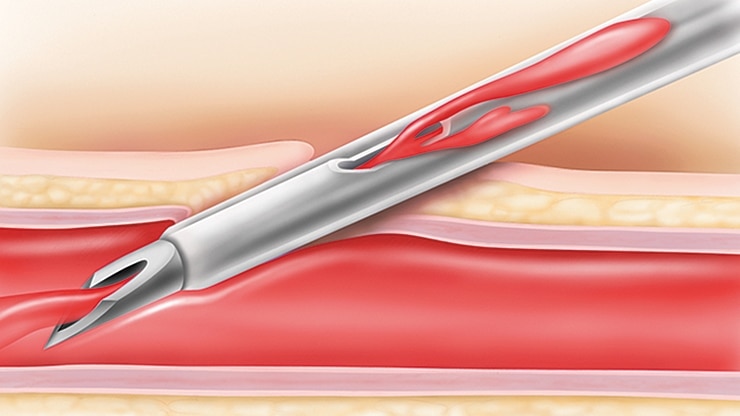
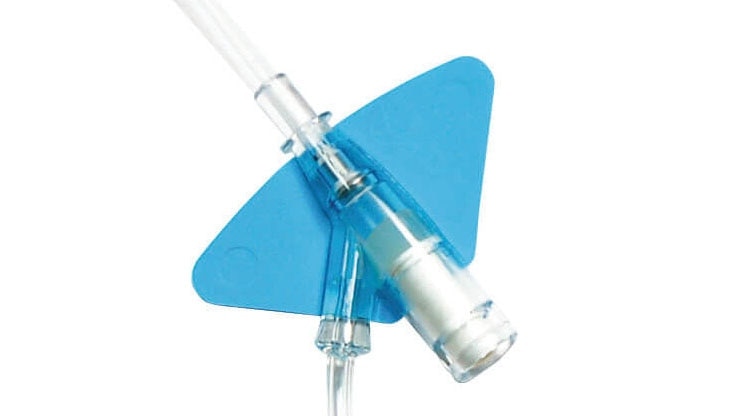
BD Nexiva* with an integrated extension tubing and stabilization platform is designed to reduce manipulation and movement at the site and has been shown to reduce dislodgement and phlebitis

The BD Nexiva* with the built-in stabilization platform reduces dislodgement by 84%1†
By choosing the BD Nexiva {*}, you can reduce the risk of complications and blood exposure as well as preserve sites*
*Compared to an open system with a PTFE catheter
The BD Nexiva {*} demonstrated a 29% reduction in phlebitis rates when compared to an open system*
*with a PTFE catheter
Dwells longer
In a randomized study comparing the BD Nexiva {*} to an open catheter system*, among peripheral IV catheters in place for over 24 hours, the median dwell time for BD Nexiva™ {*} was up to 144 hours versus 96 hours for the open system
Preserves sites
BD Nexiva {*} is an all-in-one PIVC shown to preserve sites for longer
BD Nexiva {*} has been shown to reduce costs and reduce treatment delays in clinical studies.
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD Nexiva™: Traditional vs closed IV catheter insertion
Catheter stabilization is recognized as an intervention to decrease the risk for phlebitis, catheter migration and dislodgement and may be advantageous in preventing catheter-related bloodstream infections (CRBSIs).3
Recommend limiting the use of add-on devices to reduce the potential for contamination, additional manipulation, and disconnection.5
98% reduced blood exposure during insertion due to the BD Nexiva IV catheter preassembled system.2*
Clinically demonstrated to reduce accidental dislodgement,2‡ meeting Infusion Nursing Society standards4 and CDC guidelines5 for catheter stabilization.
In a clinical study, results demonstrated a significant reduction in the rate of phlebitis (grade 2 or higher), PIVC-related complications, and infiltration in the closed system versus the open system group.1
Notes
*Compared to an open system.
**Results and savings may vary for other institutions.
†Compared with an FEP catheter.
‡When used with a specially designed 3M™ Tegaderm™ IV site securement dressing.
§Compared with B. Braun Introcan Safety® catheter with Bard Statlock® IV Ultra stabilization device.
||Compared with 96 hours in an open system.
¶Compared with a non-blood control catheter.
References
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Ann Intern Med. 1991;114(10):845-854.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2016:39(1S).
- O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. CDC. 2011:16.
BD-3595 (03/23)
